 | |
| Clinical data | |
|---|---|
| Pronunciation | /pæləˈnoʊsətrɒn/ pal-ə-NOH-sə-tron |
| Trade names | Aloxi |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a610002 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intravenous, by mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 97% (oral) |
| Protein binding | 62% |
| Metabolism | Liver, 50% (mostly CYP2D6-mediated, CYP3A4 and CYP1A2 also involved) |
| Elimination half-life | Approximately 40–50 hours |
| Excretion | Kidney, 80% (of which 49% unchanged); fecal (5 to 8%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
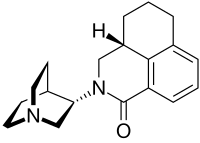
| Formula | C19H24N2O |
| Molar mass | 296.414 g·mol−1 |
| 3D model (JSmol) | |
| Specific rotation | [α]D −136° [α]D –94.1° (HCl) |
| Melting point | 87 to 88 °C (189 to 190 °F) |
| |
| |
| | |
Palonosetron, sold under the brand name Aloxi, is a medication used for the prevention and treatment of chemotherapy-induced nausea and vomiting (CINV).[2][4][5] It is a 5-HT3 antagonist.[2][4][5]
Palonosetron is administered intravenously,[6] or as a single oral capsule.[7] It has a longer duration of action than other 5-HT3 antagonists. The oral formulation was approved on August 22, 2008, for prevention of acute CINV alone, as a large clinical trial did not show oral administration to be as effective as intravenous use against delayed CINV.[7] It is on the World Health Organization's List of Essential Medicines.[8]
The oral combination netupitant/palonosetron is approved for both acute and delayed CINV.[9][10][11][12]
- ^ a b https://www.tga.gov.au/resources/auspar/auspar-palonosetron-hydrochloride [bare URL]
- ^ a b c "Aloxi 250 micrograms solution for injection - Summary of Product Characteristics (SmPC)". (emc). 18 May 2018. Archived from the original on 24 January 2022. Retrieved 23 January 2022.
- ^ "Aloxi 500 micrograms soft capsules - Summary of Product Characteristics (SmPC)". (emc). 18 May 2018. Archived from the original on 24 January 2022. Retrieved 24 January 2022.
- ^ a b c "Aloxi- palonosetron hydrochloride injection". DailyMed. Archived from the original on 24 January 2022. Retrieved 23 January 2022.
- ^ a b c "Aloxi EPAR". European Medicines Agency. 13 March 2009. Archived from the original on 14 April 2021. Retrieved 23 January 2022.
- ^ De Leon A (October 2006). "Palonosetron (Aloxi): a second-generation 5-HT₃ receptor antagonist for chemotherapy-induced nausea and vomiting". Proceedings. 19 (4): 413–6. doi:10.1080/08998280.2006.11928210. PMC 1618755. PMID 17106506.
- ^ a b Waknine Y (September 4, 2008). "FDA Approvals: Nplate, Aloxi, Vidaza". Medscape. Archived from the original on 2008-12-02. Retrieved 2008-09-04. Freely available with registration.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ "Akynzeo EPAR". European Medicines Agency. 23 June 2015. Archived from the original on 19 March 2020. Retrieved 23 January 2022.
- ^ Cite error: The named reference
EPARwas invoked but never defined (see the help page). - ^ "Akynzeo 300 mg/0.5 mg hard capsules - Summary of Product Characteristics (SmPC)". (emc). 11 February 2020. Archived from the original on 24 January 2022. Retrieved 23 January 2022.
- ^ "Akynzeo- netupitant and palonosetron capsule Akynzeo- fosnetupitant and palonosetron injection". DailyMed. Archived from the original on 18 October 2020. Retrieved 24 January 2022.