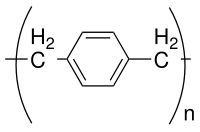

Parylene is the common name of a polymer whose backbone consists of para-benzenediyl rings −C
6H
4− connected by 1,2-ethanediyl bridges −CH
2−CH
2−. It can be obtained by polymerization of para-xylylene H
2C=C
6H
4=CH
2.
The name is also used for several polymers with the same backbone, where some hydrogen atoms are replaced by other functional groups. Some of these variants are designated in commerce by letter-number codes such as "parylene C" and "parylene AF-4". Some of these names are registered trademarks in some countries.
Coatings of parylene are often applied to electronic circuits and other equipment as electrical insulation, moisture barriers, or protection against corrosion and chemical attack (conformal coating). They are also used to reduce friction and in medicine to prevent adverse reactions to implanted devices. These coatings are typically applied by chemical vapor deposition in an atmosphere of the monomer para-xylylene.
Parylene is considered a "green" polymer because its polymerization needs no initiator or other chemicals to terminate the chain; and the coatings can be applied at or near room temperature, without any solvent.