| |
| Names | |
|---|---|
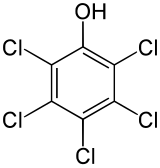
| Preferred IUPAC name
Pentachlorophenol | |
| Other names
Santophen, Pentachlorol, Chlorophen, Chlon, Dowicide 7, Pentacon, Penwar, Sinituho, Penta
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.617 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6HCl5O | |
| Molar mass | 266.34 |
| Appearance | White crystalline solid |
| Odor | benzene-like[1] |
| Density | 1.978 g/cm3 at 22 °C[2] |
| Melting point | 189.5 °C (373.1 °F; 462.6 K)[2] |
| Boiling point | 310 °C (590 °F; 583 K)[2] (decomposes) |
| 0.020 g/L at 30 °C | |
| Vapor pressure | 0.0001 mmHg (25°C)[1] |
| Thermochemistry[3] | |
Heat capacity (C)
|
202.0 J·mol−1·K−1 |
Std molar
entropy (S⦵298) |
253.2 J·mol−1·K−1 |
Std enthalpy of
formation (ΔfH⦵298) |
-292.5 kJ·mol−1 |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
117 mg/kg (mouse, oral) 128 mg/kg (hamster, oral) 17 mg/kg (rat, oral) 150 mg/kg (rat, oral)[4] |
LDLo (lowest published)
|
70 mg/kg (rabbit, oral)[4] |
LC50 (median concentration)
|
355 mg/m3 (rat) 225 mg/m3 (mouse)[4] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 0.5 mg/m3 [skin][1] |
REL (Recommended)
|
TWA 0.5 mg/m3 [skin][1] |
IDLH (Immediate danger)
|
2.5 mg/m3[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Pentachlorophenol (PCP) is an organochlorine compound used as a pesticide and a disinfectant. First produced in the 1930s, it is marketed under many trade names.[5] It can be found as pure PCP, or as the sodium salt of PCP, the latter of which dissolves easily in water. It can be biodegraded by some bacteria, including Sphingobium chlorophenolicum.
- ^ a b c d e NIOSH Pocket Guide to Chemical Hazards. "#0484". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b c Haynes, p. 3.166
- ^ Haynes, p. 5.31
- ^ a b c "Pentachlorophenol". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ "Consumer Factsheet on: Pentachlorophenol". United States Environmental Protection Agency. 2006-11-28. Retrieved 2008-02-26.