| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
2,2-Bis(hydroxymethyl)propane-1,3-diol[1] | |
| Other names
2,2-Bis(hydroxymethyl)1,3-propanediol
Pentaerythritol[1] Hercules P 6 Monopentaerythritol Tetramethylolmethane THME PETP Pentaerythrite Pentek Hercules Aqualon improved technical PE-200 | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.732 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H12O4 | |
| Molar mass | 136.15 g/mol |
| Appearance | white solid |
| Density | 1.396 g/cm3 |
| Melting point | 260.5 °C (500.9 °F; 533.6 K) |
| Boiling point | 276 °C (529 °F; 549 K) at 30 mmHg |
| |
| Solubility |
Slightly soluble in:methanol, ethanol, glycerol, ethylene glycol, formamide; insoluble in: acetone, toluene, heptane, diethyl ether, dichloromethane |
| Vapor pressure | 0.00000008 mmHg (20°C)[4] |
| Hazards | |
| Flash point | 200.1 °C (392.2 °F; 473.2 K) |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 15 mg/m3 (total) TWA 5 mg/m3 (resp)[4] |
REL (Recommended)
|
TWA 10 mg/m3 (total) TWA 5 mg/m3 (resp)[4] |
IDLH (Immediate danger)
|
N.D.[4] |
| Related compounds | |
Related compounds
|
Neopentane, Neopentyl alcohol, Neopentyl glycol, Trimethylolethane, Orthocarbonic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
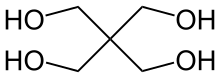
Pentaerythritol is an organic compound with the formula C(CH2OH)4. The molecular structure can be described as a neopentane with one hydrogen atom in each methyl group replaced by a hydroxyl (–OH) group. It is therefore a polyol, specifically a tetrol.
Pentaerythritol is a white solid. It is a building block for the synthesis and production of explosives, plastics, paints, appliances, cosmetics, and many other commercial products.
The word pentaerythritol is a blend of penta- in reference to its five carbon atoms and erythritol, which also possesses 4 alcohol groups.
- ^ a b Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 691. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ Yalkowsky, Samuel H. (2010). Handbook of aqueous solubility data (Second ed.). Boca Raton, FL: CRC Press. p. 185. ISBN 9781439802465.
- ^ Yadav, Manish G.; Vadgama, Rajeshkumar N.; Kavadia, Monali R.; Odaneth, Annamma Anil; Lali, Arvind M. (September 2019). "Production of Pentaerythritol Monoricinoleate (PEMR) by immobilized Candida antarctica lipase B". Biotechnology Reports. 23: e00353. doi:10.1016/j.btre.2019.e00353. PMC 6599945. PMID 31304100.
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0485". National Institute for Occupational Safety and Health (NIOSH).