 | |||
| |||
| Clinical data | |||
|---|---|---|---|
| AHFS/Drugs.com | Monograph (hydrochloride) Monograph (lactate) | ||
| Pregnancy category |
| ||
| Routes of administration | Oral, IV, IM | ||
| ATC code | |||
| Legal status | |||
| Legal status |
| ||
| Pharmacokinetic data | |||
| Bioavailability | ~20% orally | ||
| Metabolism | Hepatic | ||
| Onset of action | 15 min[2] | ||
| Elimination half-life | 2 to 3 hours | ||
| Excretion | Renal | ||
| Identifiers | |||
| |||
| CAS Number | |||
| PubChem CID | |||
| IUPHAR/BPS | |||
| DrugBank | |||
| ChemSpider | |||
| UNII | |||
| KEGG | |||
| ChEMBL | |||
| CompTox Dashboard (EPA) | |||
| ECHA InfoCard | 100.006.032 | ||
| Chemical and physical data | |||
| Formula | C19H27NO | ||
| Molar mass | 285.431 g·mol−1 | ||
| 3D model (JSmol) | |||
| |||
| |||
| (verify) | |||
Pentazocine,[3] sold under the brand name Talwin among others, is a painkiller used to treat moderate to severe pain. It is believed to work by activating (agonizing) κ-opioid receptors (KOR) and μ-opioid receptors (MOR). As such it is called an opioid as it delivers its effects on pain by interacting with the opioid receptors. It shares many of the side effects of other opioids like constipation, nausea, itching, drowsiness and respiratory depression, but unlike most other opioids it fairly frequently causes hallucinations, nightmares and delusions. It is also, unlike most other opioids, subject to a ceiling effect, which is when at a certain dose (which differs from person to person) no more pain relief is obtained by increasing the dose any further.[4]
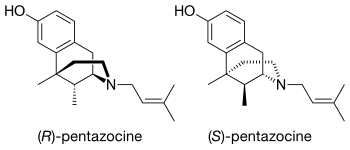
Chemically it is classed as a benzomorphan and it comes in two enantiomers, which are molecules that are exact (non-superimposable) mirror images of one another.
It was patented in 1960 and approved for medical use in 1964.[5] Usually, in its oral formulations, it is combined with naloxone so as to prevent people from crushing the tablets, dissolving them in a solvent (like water) and injecting them for a high (as orally administered naloxone produces no opioid-negating effects, whereas intravenous or intramuscular administration does).[4]
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ Stitzel RE (2004). Modern pharmacology with clinical applications (6th ed.). Philadelphia: Lippincott Williams & Wilkins. p. 325. ISBN 9780781737623.
- ^ US Patent 4105659 Analgesia producing benzazocines
- ^ a b Cite error: The named reference
martwas invoked but never defined (see the help page). - ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 527. ISBN 9783527607495.

