 | |
| Clinical data | |
|---|---|
| Trade names | Elmiron, Zycosan |
| Other names | PPS, (1->4)-β-Xylan 2,3-bis(hydrogen sulfate) with a 4 O-methyl-α-D-glucuronate |
| AHFS/Drugs.com | Monograph |
| License data | |
| Routes of administration | By mouth, intramuscular |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Excretion | Feces, urine[1] |
| Identifiers | |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| KEGG |
|
| ChEMBL |
|
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
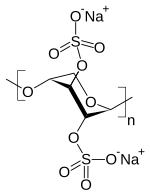
| Formula | C10H18O21S4 |
| Molar mass | 602.47 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Pentosan polysulfate, sold under the brand name Elmiron among others, is a medication used for the treatment of interstitial cystitis.[1] It was approved for medical use in the United States in 1996.[1][2][3]
- ^ a b c d "Elmiron- pentosan polysulfate sodium capsule, gelatin coated". DailyMed. 10 November 2022. Retrieved 21 December 2022.
- ^ Lindeke-Myers A, Hanif AM, Jain N (2022). "Pentosan polysulfate maculopathy". Survey of Ophthalmology. 67 (1): 83–96. doi:10.1016/j.survophthal.2021.05.005. PMID 34000253. S2CID 234767956.
- ^ "Elmiron (pentosan polysulfate sodium)" (PDF). FDA approval letter. U.S. Food and Drug Administration. 25 September 1996.