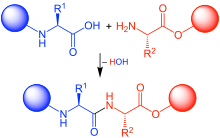
In organic chemistry, peptide synthesis is the production of peptides, compounds where multiple amino acids are linked via amide bonds, also known as peptide bonds. Peptides are chemically synthesized by the condensation reaction of the carboxyl group of one amino acid to the amino group of another. Protecting group strategies are usually necessary to prevent undesirable side reactions with the various amino acid side chains.[1] Chemical peptide synthesis most commonly starts at the carboxyl end of the peptide (C-terminus), and proceeds toward the amino-terminus (N-terminus).[2] Protein biosynthesis (long peptides) in living organisms occurs in the opposite direction.
The chemical synthesis of peptides can be carried out using classical solution-phase techniques, although these have been replaced in most research and development settings by solid-phase methods (see below).[3] Solution-phase synthesis retains its usefulness in large-scale production of peptides for industrial purposes moreover.
Chemical synthesis facilitates the production of peptides that are difficult to express in bacteria, the incorporation of unnatural amino acids, peptide/protein backbone modification, and the synthesis of D-proteins, which consist of D-amino acids.
- ^ Isidro-Llobet A, Alvarez M, Albericio F (June 2009). "Amino acid-protecting groups". Chemical Reviews. 109 (6): 2455–2504. doi:10.1021/cr800323s. hdl:2445/69570. PMID 19364121. S2CID 90409290.
- ^ Chan WC, White PD (2000). Fmoc Solid Phase Peptide Synthesis: A Practical Approach. Oxford, UK: OUP. ISBN 978-0-19-963724-9.
- ^ Jaradat DM (January 2018). "Thirteen decades of peptide synthesis: key developments in solid phase peptide synthesis and amide bond formation utilized in peptide ligation". Amino Acids. 50 (1): 39–68. doi:10.1007/s00726-017-2516-0. PMID 29185032. S2CID 3680612.