| |||
| |||
| Names | |||
|---|---|---|---|
| Systematic IUPAC name
Perchlorate[1] | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.152.366 | ||
| 2136 | |||
| MeSH | 180053 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
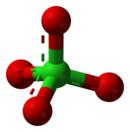
| ClO−4 | |||
| Molar mass | 99.45 g·mol−1 | ||
| Conjugate acid | Perchloric acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
A perchlorate is a chemical compound containing the perchlorate ion, ClO−4, the conjugate base of perchloric acid (ionic perchlorate). As counterions, there can be metal cations, quaternary ammonium cations or other ions, for example, nitronium cation (NO+2).
The term perchlorate can also describe perchlorate esters or covalent perchlorates.[2] These are organic compounds that are alkyl or aryl esters of perchloric acid. They are characterized by a covalent bond between an oxygen atom of the ClO4 moiety and an organyl group.
In most ionic perchlorates, the cation is non-coordinating. The majority of ionic perchlorates are commercially produced salts commonly used as oxidizers for pyrotechnic devices and for their ability to control static electricity in food packaging.[3] Additionally, they have been used in rocket propellants, fertilizers, and as bleaching agents in the paper and textile industries.
Perchlorate contamination of food and water endangers human health, primarily affecting the thyroid gland.
Ionic perchlorates are typically colorless solids that exhibit good solubility in water. The perchlorate ion forms when they dissolve in water, dissociating into ions. Many perchlorate salts also exhibit good solubility in non-aqueous solvents.[4] Four perchlorates are of primary commercial interest: ammonium perchlorate (NH4)ClO4, perchloric acid HClO4, potassium perchlorate KClO4 and sodium perchlorate NaClO4.
- ^ "Perchlorate – PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information.
- ^ Cite error: The named reference
:0was invoked but never defined (see the help page). - ^ Draft Toxicological Profile for Perchlorates, Agency for Toxic Substances and Disease Registry, U.S. Department of Health and Human Services, September, 2005.
- ^ Cite error: The named reference
Kucharzykwas invoked but never defined (see the help page).