| Peridinin-chlorophyll A binding protein | |||||||||
|---|---|---|---|---|---|---|---|---|---|
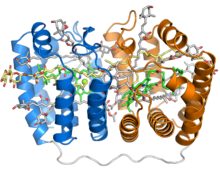 Crystal structure of the soluble peridinin-chlorophyll-protein complex from the photosynthetic dinoflagellate Amphidinium carterae. This complex is found in many photosynthetic dinoflagellates and involves a boat or cradle-shaped protein with two pseudosymmetrical repeats of eight alpha helices (shown in blue and orange) wrapped around a pigment-filled central cavity. Each eight-helix segment binds one chlorophyll molecule (green, with central magnesium ion shown as a green sphere), one diacylglycerol molecule (yellow) and four peridinin molecules (gray).[1] | |||||||||
| Identifiers | |||||||||
| Symbol | PCP | ||||||||
| Pfam | PF02429 | ||||||||
| InterPro | IPR003376 | ||||||||
| SCOP2 | 1ppr / SCOPe / SUPFAM | ||||||||
| |||||||||
The peridinin-chlorophyll-protein complex (PCP or PerCP) is a soluble molecular complex consisting of the peridinin-chlorophyll a-protein bound to peridinin, chlorophyll, and lipids. The peridinin molecules absorb light in the blue-green wavelengths (470 to 550 nm) and transfer energy to the chlorophyll molecules with extremely high efficiency.[1][2] PCP complexes are found in many photosynthetic dinoflagellates, in which they may be the primary light-harvesting complexes.[3]
- ^ a b Hofmann E, Wrench PM, Sharples FP, Hiller RG, Welte W, Diederichs K (June 1996). "Structural basis of light harvesting by carotenoids: peridinin-chlorophyll-protein from Amphidinium carterae". Science. 272 (5269): 1788–91. doi:10.1126/science.272.5269.1788. PMID 8650577.
- ^ Ghosh, S.; Bishop, M.M.; Roscioli, J.D.; LaFountain, A.M.; Frank, H.A.; Beck, W.F. (2017). "Excitation Energy Transfer by Coherent and Incoherent Mechanisms in the Peridinin-Chlorophyll a Protein". J. Phys. Chem. Lett. 8 (2): 463–469. doi:10.1021/acs.jpclett.6b02881. PMID 28042923.
- ^ Cite error: The named reference
jiang_2012was invoked but never defined (see the help page).