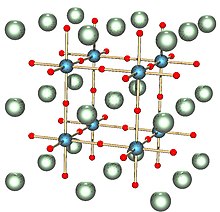



A perovskite is any material of formula ABX3 with a crystal structure similar to that of the mineral perovskite, which consists of calcium titanium oxide (CaTiO3).[2] The mineral was first discovered in the Ural mountains of Russia by Gustav Rose in 1839 and named after Russian mineralogist L. A. Perovski (1792–1856). 'A' and 'B' are two positively charged ions (i.e. cations), often of very different sizes, and X is a negatively charged ion (an anion, frequently oxide) that bonds to both cations. The 'A' atoms are generally larger than the 'B' atoms. The ideal cubic structure has the B cation in 6-fold coordination, surrounded by an octahedron of anions, and the A cation in 12-fold cuboctahedral coordination. Additional perovskite forms may exist where both/either the A and B sites have a configuration of A1x-1A2x and/or B1y-1B2y and the X may deviate from the ideal coordination configuration as ions within the A and B sites undergo changes in their oxidation states.[3]
As one of the most abundant structural families, perovskites are found in an enormous number of compounds which have wide-ranging properties, applications and importance.[4] Natural compounds with this structure are perovskite, loparite, and the silicate perovskite bridgmanite.[2][5] Since the 2009 discovery of perovskite solar cells, which contain methylammonium lead halide perovskites, there has been considerable research interest into perovskite materials.[6]
- ^ A. Navrotsky (1998). "Energetics and Crystal Chemical Systematics among Ilmenite, Lithium Niobate, and Perovskite Structures". Chem. Mater. 10 (10): 2787. doi:10.1021/cm9801901.
- ^ a b Wenk, Hans-Rudolf; Bulakh, Andrei (2004). Minerals: Their Constitution and Origin. New York, NY: Cambridge University Press. ISBN 978-0-521-52958-7.
- ^ N. Orlovskaya, N. Browning, ed. (2003). Mixed Ionic Electronic Conducting Perovskites for Advanced Energy Systems.
- ^ Artini, Cristina (2017-02-01). "Crystal chemistry, stability and properties of interlanthanide perovskites: A review". Journal of the European Ceramic Society. 37 (2): 427–440. doi:10.1016/j.jeurceramsoc.2016.08.041. ISSN 0955-2219.
- ^ Bridgemanite on Mindat.org
- ^ Fan, Zhen; Sun, Kuan; Wang, John (2015-09-15). "Perovskites for photovoltaics: a combined review of organic–inorganic halide perovskites and ferroelectric oxide perovskites". Journal of Materials Chemistry A. 3 (37): 18809–18828. doi:10.1039/C5TA04235F. ISSN 2050-7496.