 | |
 | |
| Clinical data | |
|---|---|
| Trade names | see below |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral, IM, IV |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 16,7% (rat)[5] |
| Metabolism | Hepatic — aromatic oxidation and C3-hydroxylation[6] |
| Onset of action | 1.5–4 hours |
| Elimination half-life | 6–18 hours (active metabolite unknown) |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.207.405 |
| Chemical and physical data | |
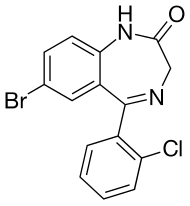
| Formula | C15H10BrClN2O |
| Molar mass | 349.61 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Phenazepam (also known in Russia as bromdihydrochlorphenylbenzodiazepine) is a benzodiazepine drug, first developed in the Soviet Union in 1975,[7] and now produced in Russia and several other countries.
Phenazepam is used in the treatment of various mental disorders such as psychiatric schizophrenia and anxiety. It can be used as a premedication before surgery as it augments the effects of anesthetics. Recently,[when?] phenazepam has gained popularity as a recreational drug; misuse has been reported in the United Kingdom,[8] Finland,[9] Sweden,[10] and the United States.[11][12]
- ^ "Therapeutic Goods (Poisons Standard—June 2024) Instrument 2024".
- ^ "Louisiana Laws - Louisiana State Legislature".
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ http://pravo.gov.ru/proxy/ips/?docbody=&nd=102119239 = СПИСОК сильнодействующих веществ для целей статьи 234 и других статей Уголовного кодекса Российской Федерации |
- ^ Pharmacokinetics of Transdermal therapeutic Systems of Phenazepam and Cytisine in the Experiment (abstract). DisserCat — Electronic Library of Dissertations (Thesis) (in Russian). 1999.
- ^ Golovenko NI, Zin'kovskiĭ VG (1979). "[2-14C-phenazepam metabolism in vitro]". Farmakologiia i Toksikologiia (in Russian). 42 (6): 597–600. PMID 40817.
- ^ [failed verification]"World Health Organization". World Health Organization. Retrieved 2017-09-20.
- ^ Corkery JM, Schifano F, Ghodse AH (May 2012). "Phenazepam". Human Psychopharmacology. 27 (3): 254–61. doi:10.1002/hup.2222. PMID 22407587. S2CID 20410292.
- ^ Kriikku P, Wilhelm L, Rintatalo J, Hurme J, Kramer J, Ojanperä I (July 2012). "Phenazepam abuse in Finland: Findings from apprehended drivers, post-mortem cases and police confiscations". Forensic Science International. 220 (1–3): 111–7. doi:10.1016/j.forsciint.2012.02.006. PMID 22391477.
- ^ Mrozkowska J, Vinge E, Borna C (2009). "[Abuse of phenazepam--new phenomenon in Sweden. Benzodiazepine derivative from Russia caused severe intoxication]". Läkartidningen. 106 (8): 516–7. PMID 19350785.
- ^ Maskell PD, De Paoli G, Nitin Seetohul L, Pounder DJ (April 2012). "Phenazepam: the drug that came in from the cold". Journal of Forensic and Legal Medicine. 19 (3): 122–5. doi:10.1016/j.jflm.2011.12.014. PMID 22390996.
- ^ Manchester KR, Waters L, Haider S, Maskell PD (July 2022). "The blood-to-plasma ratio and predicted GABAA-binding affinity of designer benzodiazepines". Forensic Toxicology. 40 (2): 349–356. doi:10.1007/s11419-022-00616-y. PMC 9715504. PMID 36454409. S2CID 247455284.