| |||
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Phenol[1] | |||
| Systematic IUPAC name
Benzenol | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.003.303 | ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2821 (solution) 2312 (molten) 1671 (solid) | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H6O | |||
| Molar mass | 94.113 g/mol | ||
| Appearance | Transparent crystalline solid | ||
| Odor | Sweet and tarry | ||
| Density | 1.07 g/cm3 | ||
| Melting point | 40.5 °C (104.9 °F; 313.6 K) | ||
| Boiling point | 181.7 °C (359.1 °F; 454.8 K) | ||
| 8.3 g/100 mL (20 °C) | |||
| log P | 1.48[2] | ||
| Vapor pressure | 0.4 mmHg (20 °C)[3] | ||
| Acidity (pKa) |
| ||
| Conjugate base | Phenoxide | ||
| UV-vis (λmax) | 270.75 nm[5] | ||
| 1.224 D | |||
| Pharmacology | |||
| C05BB05 (WHO) D08AE03 (WHO), N01BX03 (WHO), R02AA19 (WHO) | |||
| Hazards | |||
| GHS labelling: | |||
   [6] [6]
| |||
| Danger | |||
| H301, H311, H314, H331, H341, H373[6] | |||
| P261, P280, P301+P310, P305+P351+P338, P310[6] | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 79 °C (174 °F; 352 K) | ||
| Explosive limits | 1.8–8.6%[3] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
| ||
LDLo (lowest published)
|
| ||
LC50 (median concentration)
|
| ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 5 ppm (19 mg/m3) [skin][3] | ||
REL (Recommended)
|
| ||
IDLH (Immediate danger)
|
250 ppm[3] | ||
| Safety data sheet (SDS) | [1] | ||
| Related compounds | |||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
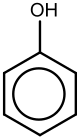
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula C6H5OH.[5] It is a white crystalline solid that is volatile. The molecule consists of a phenyl group (−C6H5) bonded to a hydroxy group (−OH). Mildly acidic, it requires careful handling because it can cause chemical burns.[5]
Phenol was first extracted from coal tar, but today is produced on a large scale (about 7 million tonnes a year) from petroleum-derived feedstocks. It is an important industrial commodity as a precursor to many materials and useful compounds.[8] It is primarily used to synthesize plastics and related materials. Phenol and its chemical derivatives are essential for production of polycarbonates, epoxies, explosives, Bakelite, nylon, detergents, herbicides such as phenoxy herbicides, and numerous pharmaceutical drugs.[9]
- ^ "Front Matter". Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 690. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
Only one name is retained, phenol, for C6H5-OH, both as a preferred name and for general nomenclature.
- ^ "Phenol_msds".
- ^ a b c d e NIOSH Pocket Guide to Chemical Hazards. "#0493". National Institute for Occupational Safety and Health (NIOSH).
- ^ Kütt, Agnes; Movchun, Valeria; Rodima, Toomas; et al. (2008). "Pentakis(trifluoromethyl)phenyl, a Sterically Crowded and Electron-withdrawing Group: Synthesis and Acidity of Pentakis(trifluoromethyl)benzene, -toluene, -phenol, and -aniline". The Journal of Organic Chemistry. 73 (7): 2607–20. doi:10.1021/jo702513w. PMID 18324831.
- ^ a b c "Phenol". PubChem, US National Library of Medicine. 10 June 2023. Retrieved 12 June 2023.
- ^ a b c Sigma-Aldrich Co., Phenol. Retrieved on 2022-02-15.
- ^ a b c "Phenol". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Weber, Manfred; Weber, Markus; Kleine-Boymann, Michael (2004). "Phenol". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_299.pub3. ISBN 978-3527306732.
- ^ Zvi Rappoport, ed. (2003). The Chemistry of Phenols. PATAI'S Chemistry of Functional Groups. John Wiley & Sons. doi:10.1002/0470857277. ISBN 9780470857274.