 | |||
| |||
| Clinical data | |||
|---|---|---|---|
| Trade names | Adipex-P, Ionamin, Suprenza, others | ||
| Other names | α-methyl-amphetamine α,α-dimethylphenethylamine | ||
| AHFS/Drugs.com | Monograph | ||
| MedlinePlus | a682187 | ||
| Pregnancy category |
| ||
| Dependence liability | Physical: Not typical Psychological: Moderate[1] | ||
| Addiction liability | Low[2] | ||
| Routes of administration | By mouth | ||
| Drug class | Appetite suppressant[3] | ||
| ATC code | |||
| Legal status | |||
| Legal status |
| ||
| Pharmacokinetic data | |||
| Bioavailability | High (almost complete)[5] | ||
| Protein binding | Approximately 96.3% | ||
| Metabolism | Liver[5] | ||
| Elimination half-life | 25 hours, urinary pH-dependent[5] | ||
| Excretion | Kidney (62–85% unchanged)[5] | ||
| Identifiers | |||
| |||
| CAS Number | |||
| PubChem CID | |||
| IUPHAR/BPS | |||
| DrugBank | |||
| ChemSpider | |||
| UNII | |||
| KEGG | |||
| ChEBI | |||
| ChEMBL | |||
| CompTox Dashboard (EPA) | |||
| ECHA InfoCard | 100.004.112 | ||
| Chemical and physical data | |||
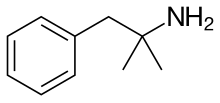
| Formula | C10H15N | ||
| Molar mass | 149.237 g·mol−1 | ||
| 3D model (JSmol) | |||
| |||
| |||
| (verify) | |||
Phentermine, sold under the brand name Adipex-P among others, is a medication used together with diet and exercise to treat obesity.[3] It is available by itself or as the combination phentermine/topiramate.[6]
Common side effects include a fast heart beat, high blood pressure, trouble sleeping, dizziness, and restlessness.[3] Serious side effects may include abuse, but do not include pulmonary hypertension or valvular heart disease, as the latter were caused by the fenfluramine component of the fen-phen drug combination.[3] It works mainly as an appetite suppressant, likely as a result of being a central nervous system (CNS) stimulant.[3] Chemically, phentermine is a substituted amphetamine.[7]
Phentermine was approved for medical use in the United States in 1959.[3] It is available as a generic medication.[3] In 2022, it was the 149th most commonly prescribed medication in the United States, with more than 3 million prescriptions.[8][9] Phentermine was withdrawn from the market in the United Kingdom in 2000, while the combination medication fen-phen, of which it was a part, was withdrawn from the market in 1997 due to side effects of fenfluramine.[10]
- ^ Tarascon Pocket Pharmacopoeia 2017 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. 2016. p. 7. ISBN 9781284118971.
- ^ Sadock BJ, Sadock VA (2010). Kaplan and Sadock's Pocket Handbook of Clinical Psychiatry. Lippincott Williams & Wilkins. p. 435. ISBN 9781605472645.
- ^ a b c d e f g "Phentermine Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 13 April 2019.
- ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 16 August 2023.
- ^ a b c d Cite error: The named reference
TGAwas invoked but never defined (see the help page). - ^ "Phentermine and topiramate Uses, Side Effects & Warnings". Drugs.com. Retrieved 13 April 2019.
- ^ Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ (July 2012). "Biosynthesis of amphetamine analogs in plants". Trends in Plant Science. 17 (7): 404–412. Bibcode:2012TPS....17..404H. doi:10.1016/j.tplants.2012.03.004. PMID 22502775.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Phentermine Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- ^ Bagchi D, Preuss HG (2012). Obesity: Epidemiology, Pathophysiology, and Prevention (Second ed.). CRC Press. p. 314. ISBN 9781439854259.

