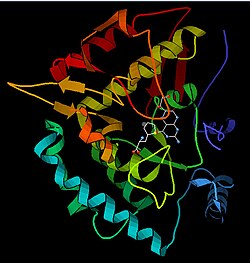
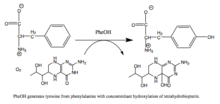
Phenylalanine hydroxylase (PAH) (EC 1.14.16.1) is an enzyme that catalyzes the hydroxylation of the aromatic side-chain of phenylalanine to generate tyrosine. PAH is one of three members of the biopterin-dependent aromatic amino acid hydroxylases, a class of monooxygenase that uses tetrahydrobiopterin (BH4, a pteridine cofactor) and a non-heme iron for catalysis. During the reaction, molecular oxygen is heterolytically cleaved with sequential incorporation of one oxygen atom into BH4 and phenylalanine substrate.[5][6] In humans, mutations in its encoding gene, PAH, can lead to the metabolic disorder phenylketonuria.
 |
- ^ a b c GRCh38: Ensembl release 89: ENSG00000171759 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000020051 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Fitzpatrick PF (1999). "Tetrahydropterin-dependent amino acid hydroxylases". Annual Review of Biochemistry. 68: 355–81. doi:10.1146/annurev.biochem.68.1.355. PMID 10872454.
- ^ Kaufman S (February 1958). "A new cofactor required for the enzymatic conversion of phenylalanine to tyrosine". The Journal of Biological Chemistry. 230 (2): 931–9. doi:10.1016/S0021-9258(18)70516-4. PMID 13525410.