| |

| |
| Names | |
|---|---|
| Systematic IUPAC name
Phenyllithium[2] | |
| Other names
Lithiobenzene[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | LiPh, PhLi |
| 506502 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.838 |
| EC Number |
|
| 2849 | |
| MeSH | phenyllithium |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
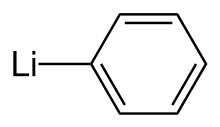
| LiC 6H 5 | |
| Molar mass | 84.045 g mol−1 |
| Appearance | Colorless crystals |
| Density | 828 mg cm−3 |
| Boiling point | 140 to 143 °C (284 to 289 °F; 413 to 416 K) |
| Reacts | |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
48.3-52.5 kJ mol−1 |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H226, H250, H261, H302, H312, H314, H332 | |
| P210, P222, P231+P232, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P280, P301+P312, P301+P330+P331, P302+P334, P302+P352, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P310, P312, P321, P322, P330, P363, P370+P378, P402+P404, P403+P235, P405, P422, P501 | |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Related compounds
|
phenylcopper, phenylsodium, phenylcobalt |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Phenyllithium is an organometallic agent with the empirical formula C6H5Li. It is most commonly used as a metalating agent in organic syntheses and a substitute for Grignard reagents for introducing phenyl groups in organic syntheses.[3] Crystalline phenyllithium is colorless; however, solutions of phenyllithium are various shades of brown or red depending on the solvent used and the impurities present in the solute.[4]
- ^ Typically used to describe substituted derivatives. See, e.g., Katsutoshi Kobayashi; Soichi Sato; Horn, Ernst; Naomichi Furukawa (1998), "First isolation and characterization of sulfenium cation salts stabilized by the coordination of two nitrogen atoms," Tetrahedron Letters, 39: 17, pp. 2593-2596. ISSN 0040-4039. DOI 10.1016/S0040-4039(98)00277-9.
- ^ "phenyllithium (CHEBI:51470)". Chemical Entities of Biological Interest (ChEBI). Cambridge, UK: European Bioinformatics Institute. 2009-01-22. Main. Retrieved 2013-06-01.
- ^ Wietelmann, U.; Bauer, R. J. "Lithium and Lithium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a15_393. ISBN 978-3527306732.
- ^ Gilman, H.; Zoellner, E. A.; Selby, W. M. (1932). "An Improved Procedure for the Preparation of Organolithium Compounds". Journal of the American Chemical Society. 54 (5): 1957–1962. doi:10.1021/ja01344a033.