| |

| |
 A sample case of toxic gases used in chemical warfare; the leftmost contains phosgene in a sealed capillary
| |
| Names | |
|---|---|
| Preferred IUPAC name
Carbonyl dichloride[2] | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.000.792 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1076 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| COCl2 | |
| Molar mass | 98.91 g·mol−1 |
| Appearance | Colorless gas |
| Odor | Suffocating, like musty hay or grass[3] |
| Density | 4.248 g/L (15 °C, gas) 1.432 g/cm3 (0 °C, liquid) |
| Melting point | −118 °C (−180 °F; 155 K) |
| Boiling point | 8.3 °C (46.9 °F; 281.4 K) |
| Insoluble, reacts[4] | |
| Solubility | Soluble in benzene, toluene, acetic acid Decomposes in alcohol and acid |
| Vapor pressure | 1.6 atm (20°C)[3] |
| −48·10−6 cm3/mol | |
| Structure | |
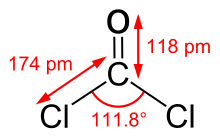
| Trigonal planar | |
| 1.17 D | |
| Hazards | |
| GHS labelling: | |
  [5] [5]
| |
| Danger | |
| H314, H330[5] | |
| P260, P280, P303+P361+P353+P315, P304+P340+P315, P305+P351+P338+P315, P403, P405[5] | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
Threshold limit value (TLV)
|
0.1 ppm (1 ppm = 4 mg/m3) |
| Lethal dose or concentration (LD, LC): | |
LC50 (median concentration)
|
|
LCLo (lowest published)
|
|
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 0.1 ppm (0.4 mg/m3)[3] |
REL (Recommended)
|
TWA 0.1 ppm (0.4 mg/m3) C 0.2 ppm (0.8 mg/m3) [15-minute][3] |
IDLH (Immediate danger)
|
2 ppm[3] 1 ppm = 4 mg/m3 |
| Safety data sheet (SDS) | [1] |
| Related compounds | |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Phosgene is an organic chemical compound with the formula COCl2. It is a toxic, colorless gas; in low concentrations, its musty odor resembles that of freshly cut hay or grass.[7] It can be thought of chemically as the double acyl chloride analog of carbonic acid, or structurally as formaldehyde with the hydrogen atoms replaced by chlorine atoms. Phosgene is a valued and important industrial building block, especially for the production of precursors of polyurethanes and polycarbonate plastics.
Phosgene is extremely poisonous and was used as a chemical weapon during World War I, where it was responsible for 85,000 deaths. It is a highly potent pulmonary irritant and quickly filled enemy trenches due to it being a heavy gas.
It is classified as a Schedule 3 substance under the Chemical Weapons Convention. In addition to its industrial production, small amounts occur from the breakdown and the combustion of organochlorine compounds, such as chloroform.[8]
- ^ Merck Index, 11th Edition, 7310.
- ^ Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: Royal Society of Chemistry. 2014. p. 798. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ a b c d e NIOSH Pocket Guide to Chemical Hazards. "#0504". National Institute for Occupational Safety and Health (NIOSH).
- ^ "PHOSGENE (cylinder)". Inchem (Chemical Safety Information from Intergovernmental Organizations). International Programme on Chemical Safety and the European Commission.
- ^ a b c Record of Phosgene in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 16 March 2021.
- ^ a b "Phosgene". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ CBRNE - Lung-Damaging Agents, Phosgene May 27, 2009
- ^ Wolfgang Schneider; Werner Diller. "Phosgene". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_411. ISBN 978-3527306732.
