| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Phosphate[1]
| |||
| Other names
Orthophosphate
Tetraoxophosphate(V) Tetraoxidophosphate(V) | |||
| Identifiers | |||
3D model (JSmol)
|
| ||
| 3903772 | |||
| ChEBI | |||
| ChemSpider | |||
| 1997 | |||
| MeSH | Phosphates | ||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| PO3− 4 | |||
| Molar mass | 94.9714 g mol−1 | ||
| Conjugate acid | Monohydrogen phosphate | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
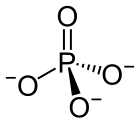
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid, a.k.a. phosphoric acid H3PO4.
The phosphate or orthophosphate ion [PO
4]3−
is derived from phosphoric acid by the removal of three protons H+
. Removal of one proton gives the dihydrogen phosphate ion [H
2PO
4]−
while removal of two protons gives the hydrogen phosphate ion [HPO
4]2−
. These names are also used for salts of those anions, such as ammonium dihydrogen phosphate and trisodium phosphate.
-
[PO
4]3−
Phosphate or orthophosphate
In organic chemistry, phosphate or orthophosphate is an organophosphate, an ester of orthophosphoric acid of the form PO
4RR′R″ where one or more hydrogen atoms are replaced by organic groups. An example is trimethyl phosphate, (CH
3)
3PO
4. The term also refers to the trivalent functional group OP(O-)
3 in such esters. Phosphates may contain sulfur in place of one or more oxygen atoms (thiophosphates and organothiophosphates).
Orthophosphates are especially important among the various phosphates because of their key roles in biochemistry, biogeochemistry, and ecology, and their economic importance for agriculture and industry.[2] The addition and removal of phosphate groups (phosphorylation and dephosphorylation) are key steps in cell metabolism.
Orthophosphates can condense to form pyrophosphates.
- ^ "Phosphates – PubChem Public Chemical Database". The PubChem Project. USA: National Center of Biotechnology Information.
- ^ "Phosphate Primer". Florida Industrial and Phosphate Research Institute. Florida Polytechnic University. Archived from the original on 29 August 2017. Retrieved 30 March 2018.


![[H 2PO 4]− Dihydrogen phosphate](http://upload.wikimedia.org/wikipedia/commons/thumb/d/db/2-dihydrogenphosphate-3D-balls.png/209px-2-dihydrogenphosphate-3D-balls.png)
![[HPO 4]2− Hydrogen phosphate](http://upload.wikimedia.org/wikipedia/commons/thumb/8/87/1-hydrogenphosphate-3D-balls.png/169px-1-hydrogenphosphate-3D-balls.png)
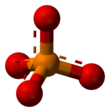
![[PO 4]3− Phosphate or orthophosphate](http://upload.wikimedia.org/wikipedia/commons/thumb/b/b0/0-phosphate-3D-balls.png/165px-0-phosphate-3D-balls.png)