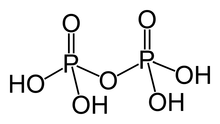
In chemistry, a phosphoric acid, in the general sense, is a phosphorus oxoacid in which each phosphorus (P) atom is in the oxidation state +5, and is bonded to four oxygen (O) atoms, one of them through a double bond, arranged as the corners of a tetrahedron. Two or more of these PO4 tetrahedra may be connected by shared single-bonded oxygens, forming linear or branched chains, cycles, or more complex structures. The single-bonded oxygen atoms that are not shared are completed with acidic hydrogen atoms. The general formula of a phosphoric acid is Hn+2−2xPnO3n+1−x, where n is the number of phosphorus atoms and x is the number of fundamental cycles in the molecule's structure, between 0 and n + 2/2.

Removal of protons (H+) from k hydroxyl groups –OH leaves anions generically called phosphates (if k = n − 2x + 2) or hydrogen phosphates (if k is between 1 and n − 2x + 1), with general formula [Hn−2x+2−kPnO3n+1−x]k−. The fully dissociated anion (k = n − 2x + 2) has formula [PnO3n−x+1](n−2x+2)−. The term phosphate is also used in organic chemistry for the functional groups that result when one or more of the hydrogens are replaced by bonds to other groups.
These acids, together with their salts and esters, include some of the best-known compounds of phosphorus, of high importance in biochemistry, mineralogy, agriculture, pharmacy, chemical industry, and chemical research.