| |

| |

| |
| Names | |
|---|---|
| IUPAC name
Phosphorus trichloride
| |
| Systematic IUPAC name
Trichlorophosphane | |
| Other names
Phosphorus(III) chloride
Phosphorous chloride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.028.864 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1809 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
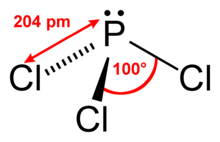
| PCl3 | |
| Molar mass | 137.33 g/mol |
| Appearance | Colorless to yellow fuming liquid[1] |
| Odor | unpleasant, acrid, like hydrochloric acid[1] |
| Density | 1.574 g/cm3 |
| Melting point | −93.6 °C (−136.5 °F; 179.6 K) |
| Boiling point | 76.1 °C (169.0 °F; 349.2 K) |
| hydrolyzes | |
| Solubility in other solvents | soluble[vague] in benzene, CS2, ether, chloroform, CCl4, halogenated organic solvents reacts with ethanol |
| Vapor pressure | 13.3 kPa |
| −63.4·10−6 cm3/mol | |
Refractive index (nD)
|
1.5122 (21 °C) |
| Viscosity | 0.65 cP (0 °C) 0.438 cP (50 °C) |
| 0.97 D | |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
−319.7 kJ/mol |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Highly toxic,[2] corrosive |
| GHS labelling:[4] | |
  
| |
| Danger | |
| H300, H301, H314, H330, H373 | |
| P260, P273, P284, P303+P361+P353, P304+P340+P310, P305+P351+P338 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
18 mg/kg (rat, oral)[3] |
LC50 (median concentration)
|
104 ppm (rat, 4 hr) 50 ppm (guinea pig, 4 hr)[3] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 0.5 ppm (3 mg/m3)[1] |
REL (Recommended)
|
TWA 0.2 ppm (1.5 mg/m3) ST 0.5 ppm (3 mg/m3)[1] |
IDLH (Immediate danger)
|
25 ppm[1] |
| Safety data sheet (SDS) | ICSC 0696 |
| Related compounds | |
Related phosphorus chlorides
|
Phosphorus pentachloride Phosphorus oxychloride Diphosphorus tetrachloride |
Related compounds
|
Phosphorus trifluoride Phosphorus tribromide Phosphorus triiodide |
| Supplementary data page | |
| Phosphorus trichloride (data page) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Phosphorus trichloride is an inorganic compound with the chemical formula PCl3. A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic and reacts readily with water to release hydrogen chloride.
- ^ a b c d e NIOSH Pocket Guide to Chemical Hazards. "#0511". National Institute for Occupational Safety and Health (NIOSH).
- ^ Phosphorus trichloride toxicity
- ^ a b "Phosphorus trichloride". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Sigma-Aldrich Co., Phosphorus trichloride.
