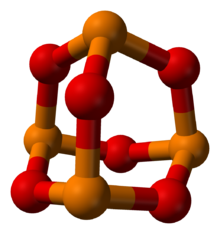
 Phosphorus in orange, oxygen in red
| |

| |
| Names | |
|---|---|
| IUPAC names
Tetraphosphorus hexaoxide
Tricyclo[3.3.1.13,7]tetraphosphoxane | |
| Systematic IUPAC name
2,4,6,8,9,10-Hexaoxa-1,3,5,7-tetraphosphatricyclo[3.3.1.13,7]decane | |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.032.414 |
| EC Number |
|
| 26856 | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| P4O6 | |
| Molar mass | 219.88 g mol−1 |
| Appearance | colourless monoclinic crystals or liquid |
| Density | 2.135 g/cm3 |
| Melting point | 23.8 °C (74.8 °F; 296.9 K) |
| Boiling point | 173.1 °C (343.6 °F; 446.2 K) |
| reacts | |
| Acidity (pKa) | 9.4 |
| Structure | |
| See Text | |
| 0 | |
| Hazards | |
| GHS labelling: | |

| |
| Danger | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions
|
Phosphorus trisulfide |
Other cations
|
Dinitrogen trioxide Arsenic trioxide Antimony trioxide |
Related compounds
|
Phosphorus pentoxide Phosphorous acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Phosphorus trioxide is the chemical compound with the molecular formula P4O6. Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. This colorless solid is structurally related to adamantane. It is formally the anhydride of phosphorous acid, H3PO3, but cannot be obtained by the dehydration of the acid. A white solid that melts at room temperature, it is waxy, crystalline and highly toxic, with garlic odor.[1]
- ^ A. F. Holleman; Wiberg, Egon; Wiberg, Nils (2001). Inorganic Chemistry. Boston: Academic Press. ISBN 0-12-352651-5.
