This article needs additional citations for verification. (June 2019) |
Polarography is a type of voltammetry where the working electrode is a dropping mercury electrode (DME) or a static mercury drop electrode (SMDE), which are useful for their wide cathodic ranges and renewable surfaces. It was invented in 1922 by Czechoslovak chemist Jaroslav Heyrovský, for which he won the Nobel prize in 1959.[1][2][3][4][5][6] The main advantages of mercury as electrode material are as follows: 1) a large voltage window: ca. from +0.2 V to -1.8 V vs reversible hydrogen electrode (RHE). Hg electrode is particularly well-suited for studying electroreduction reactions. 2) very reproducible electrode surface, since mercury is liquid. 3) very easy cleaning of the electrode surface by making a new drop of mercury from a large Hg pool connected by a glass capillary.
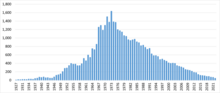
Polarography played a major role as an experimental tool in the advancement of both Analytical Chemistry and Electrochemistry until the 1990s (see figure below), when it was supplanted by other methods that did not require the use of mercury.
- ^ Reinmuth, W. H. (1961-11-01). "Theory of Stationary Electrode Polarography". Analytical Chemistry. 33 (12): 1793–1794. doi:10.1021/ac60180a004.
- ^ Nicholson, R. S.; Irving. Shain (1964-04-01). "Theory of Stationary Electrode Polarography. Single Scan and Cyclic Methods Applied to Reversible, Irreversible, and Kinetic Systems". Analytical Chemistry. 36 (4): 706–723. doi:10.1021/ac60210a007.
- ^ Skoog, Douglas A.; Donald M. West; F. James Holler (1995-08-25). Fundamentals of Analytical Chemistry (7th ed.). Harcourt Brace College Publishers. ISBN 978-0-03-005938-4.
- ^ Kissinger, Peter; William R. Heineman (1996-01-23). Laboratory Techniques in Electroanalytical Chemistry, Second Edition, Revised and Expanded (2 ed.). CRC. ISBN 978-0-8247-9445-3.
- ^ Bard, Allen J.; Larry R. Faulkner (2000-12-18). Electrochemical Methods: Fundamentals and Applications (2 ed.). Wiley. ISBN 978-0-471-04372-0.
- ^ Zoski, Cynthia G. (2007-02-07). Handbook of Electrochemistry. Elsevier Science. ISBN 978-0-444-51958-0.