Skeletal formula of trans-polyacetylene
| |
Skeletal formula of cis-polyacetylene
| |
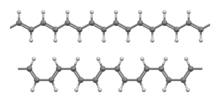 Ball-and-stick models of the transoidal (top) and cisoidal (bottom) conformations of the trans isomer[1]
| |
| Names | |
|---|---|
| IUPAC name
Polyethyne
| |
| Other names
Polyacetylene, PAc
| |
| Identifiers | |
| ChemSpider |
|
| Properties | |
| [C2H2]n | |
| insoluble | |
| Related compounds | |
Related compounds
|
Acetylene gas (monomer) |
| Hazards | |
| GHS labelling: | |

| |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Polyacetylene (IUPAC name: polyethyne) usually refers to an organic polymer with the repeating unit [C2H2]n. The name refers to its conceptual construction from polymerization of acetylene to give a chain with repeating olefin groups. This compound is conceptually important, as the discovery of polyacetylene and its high conductivity upon doping helped to launch the field of organic conductive polymers. The high electrical conductivity discovered by Hideki Shirakawa, Alan Heeger, and Alan MacDiarmid for this polymer led to intense interest in the use of organic compounds in microelectronics (organic semiconductors). This discovery was recognized by the Nobel Prize in Chemistry in 2000.[2][3] Early work in the field of polyacetylene research was aimed at using doped polymers as easily processable and lightweight "plastic metals".[4] Despite the promise of this polymer in the field of conductive polymers, many of its properties such as instability to air and difficulty with processing have led to avoidance in commercial applications.
Compounds called polyacetylenes also occur in nature, although in this context the term refers to polyynes, compounds containing multiple acetylene groups ("poly" meaning many), rather than to chains of olefin groups ("poly" meaning polymerization of).[5]
- ^ Perego, Giovanni; Lugli, Gabriele; Pedretti, Ugo; Cesari, Marco (1988). "X-ray investigation on highly oriented polyacetylene, 1. Crystal structure of cis- and trans-polyacetylene". Makromol. Chem. 189 (11): 2657–2669. doi:10.1002/macp.1988.021891113.
- ^ Heeger, Alan (2001). "Nobel Lecture: Semiconducting and metallic polymers: The fourth generation of polymeric materials". Reviews of Modern Physics. 73 (3): 681–700. Bibcode:2001RvMP...73..681H. doi:10.1103/RevModPhys.73.681.
- ^ "The Nobel Prize in Chemistry 2000".
- ^ Cite error: The named reference
Grubbswas invoked but never defined (see the help page). - ^ Minto, Robert E. (2008). "Biosynthesis and function of polyacetylenes and allied natural products". Progress in Lipid Research. 47 (4): 233–306. doi:10.1016/j.plipres.2008.02.002. PMC 2515280. PMID 18387369..