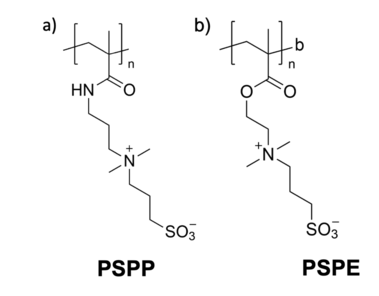
Polysulfobetaines are zwitterionic polymers that contain a positively charged quaternary ammonium and a negatively charged sulfonate group within one constitutional repeat unit.[1][2] In recent years, polysulfobetaines have received increasing attention owing to their good biotolerance and ultralow-fouling behavior towards surfaces. These properties are mainly referred to a tightly bound hydration layer around each zwitterionic group, which effectively suppresses protein adsorption and thus, improves anti-fouling behavior.[3][4] Therefore, polysulfobetaines have been typically employed as ultrafiltration membranes,[4] blood-contacting devices,[5] and drug delivery materials.[3]
The chemical structure of polysulfobetaines can be divided in several subgroups. Most widespread are amides of (meth)acrylic acid ('PSPP') or quaternary esters ('PSPE'). Also, compounds from poly(vinylpyridinium), poly(vinylimidazolium), or quaternary poly(pyrrolidinium) as well as zwitterionic ionenes, are often found.[2][6][7][8][9][10]
- ^ Lowe, Andrew B.; McCormick, Charles L. (2002-11-01). "Synthesis and Solution Properties of Zwitterionic Polymers". Chemical Reviews. 102 (11): 4177–4190. doi:10.1021/cr020371t. ISSN 0009-2665. PMID 12428987.
- ^ a b Laschewsky, André (2014-05-23). "Structures and Synthesis of Zwitterionic Polymers". Polymers. 6 (5): 1544–1601. doi:10.3390/polym6051544. ISSN 2073-4360.
- ^ a b Woodfield, Peter A.; Zhu, Yicheng; Pei, Yiwen; Roth, Peter J. (2014-01-28). "Hydrophobically Modified Sulfobetaine Copolymers with Tunable Aqueous UCST through Postpolymerization Modification of Poly(pentafluorophenyl acrylate)". Macromolecules. 47 (2): 750–762. Bibcode:2014MaMol..47..750W. doi:10.1021/ma402391a. hdl:20.500.11937/3990. ISSN 0024-9297.
- ^ a b Wu, Jiang; Lin, Weifeng; Wang, Zhen; Chen, Shengfu; Chang, Yung (2012-05-15). "Investigation of the Hydration of Nonfouling Material Poly(sulfobetaine methacrylate) by Low-Field Nuclear Magnetic Resonance". Langmuir. 28 (19): 7436–7441. doi:10.1021/la300394c. ISSN 0743-7463. PMID 22512533.
- ^ Yuan, Jiang; Huang, Xiaobo; Li, Pengfei; Li, Li; Shen, Jian (2013-08-28). "Surface-initiated RAFT polymerization of sulfobetaine from cellulose membranes to improve hemocompatibility and antibiofouling property". Polymer Chemistry. 4 (19): 5074–5085. doi:10.1039/C3PY00565H. ISSN 1759-9962.
- ^ Kudaibergenov, Sarkyt; Jaeger, Werner; Laschewsky, Andre (2006), "Polymeric Betaines: Synthesis, Characterization, and Application", Supramolecular Polymers Polymeric Betains Oligomers, vol. 201, Berlin, Heidelberg: Springer Berlin Heidelberg, pp. 157–224, doi:10.1007/12_078, ISBN 978-3-540-31923-8, retrieved 2022-02-22
- ^ Tarannum, Nazia; Singh, Meenakshi (2013-06-01). "Advances in Synthesis and Applications of Sulfo and Carbo Analogues of Polybetaines: A Review". Reviews in Advanced Sciences and Engineering. 2 (2): 90–111. doi:10.1166/rase.2013.1036. ISSN 2157-9121.
- ^ Wielema, T. A.; Engberts, J. B. F. N. (1987). "Zwitterionic polymers—I. Synthesis of a novel series of poly(vinylsulphobetaines). Effect of structure of polymer on solubility in water". European Polymer Journal. 23 (12): 947–950. Bibcode:1987EurPJ..23..947W. doi:10.1016/0014-3057(87)90038-3. ISSN 0014-3057.
- ^ Grassl, B.; Galin, J.C. (1995). "Segmented Poly(tetramethylene oxide) Zwitterionomers and Their Homologous Ionenes. 1. Synthesis, Molecular Characterization, and Thermal Stability". Macromolecules. 28 (21): 7035–7045. Bibcode:1995MaMol..28.7035G. doi:10.1021/ma00125a001.
- ^ Grassl, Bruno; Meurer, Bernard; Scheer, Monique; Galin, Jean Claude (1997-01-01). "Segmented Poly(tetramethylene oxide) Zwitterionomers and Their Homologous Ionenes. 2. Phase Separation through DSC and Solid State 1 H-NMR Spectroscopy". Macromolecules. 30 (2): 236–245. Bibcode:1997MaMol..30..236G. doi:10.1021/ma960643s. ISSN 0024-9297.