


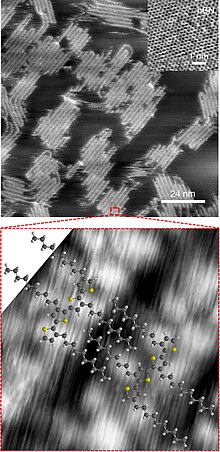
Polythiophenes (PTs) are polymerized thiophenes, a sulfur heterocycle. The parent PT is an insoluble colored solid with the formula (C4H2S)n.[notes 1][2][3] The rings are linked through the 2- and 5-positions. Poly(alkylthiophene)s have alkyl substituents at the 3- or 4-position(s). They are also colored solids, but tend to be soluble in organic solvents.
PTs become conductive when oxidized. The electrical conductivity results from the delocalization of electrons along the polymer backbone. Conductivity however is not the only interesting property resulting from electron delocalization. The optical properties of these materials respond to environmental stimuli, with dramatic color shifts in response to changes in solvent, temperature, applied potential, and binding to other molecules. Changes in both color and conductivity are induced by the same mechanism, twisting of the polymer backbone and disrupting conjugation, making conjugated polymers attractive as sensors that can provide a range of optical and electronic responses.[4][5][6]
The development of polythiophenes and related conductive organic polymers was recognized by the awarding of the 2000 Nobel Prize in Chemistry to Alan J. Heeger, Alan MacDiarmid, and Hideki Shirakawa "for the discovery and development of conductive polymers".
- ^ Arosio, Paolo; Moreno, Margherita; Famulari, Antonino; Raos, Guido; Catellani, Marinella; Valdo Meille, Stefano (2009). "Ordered Stacking of Regioregular Head-to-Tail Polyalkylthiophenes: Insights from the Crystal Structure of Form I′ Poly(3-n-butylthiophene)". Chem. Mater. 21 (1): 78–87. doi:10.1021/cm802168e.
- ^ Tourillon, G.; Garnier, F. (April 1982). "New electrochemically generated organic conducting polymers". Journal of Electroanalytical Chemistry and Interfacial Electrochemistry. 135 (1): 173–178. doi:10.1016/0022-0728(82)90015-8.
- ^ Österholm, J.-E.; Passiniemi, P.; Isotalo, H.; Stubb, H. (February 1987). "Synthesis and properties of FeCl4-doped polythiophene". Synthetic Metals. 18 (1–3): 213–218. doi:10.1016/0379-6779(87)90881-2.
- ^ Nielsen, Christian B.; McCulloch, Iain (2013). "Recent advances in transistor performance of polythiophenes". Progress in Polymer Science. 38 (12): 2053–2069. doi:10.1016/j.progpolymsci.2013.05.003. hdl:10044/1/14442. S2CID 136757919.
- ^ McQuade, D. Tyler; Pullen, Anthony E.; Swager, Timothy M. (2000). "Conjugated Polymer-Based Chemical Sensors". Chemical Reviews. 100 (7): 2537–74. doi:10.1021/cr9801014. PMID 11749295. S2CID 4936796.
- ^ Mehmood, Umer; Al-Ahmed, Amir; Hussein, Ibnelwaleed A. (2016). "Review on recent advances in polythiophene based photovoltaic devices". Renewable & Sustainable Energy Reviews. 57: 550–561. Bibcode:2016RSERv..57..550M. doi:10.1016/j.rser.2015.12.177. S2CID 101640805.
Cite error: There are <ref group=notes> tags on this page, but the references will not show without a {{reflist|group=notes}} template (see the help page).