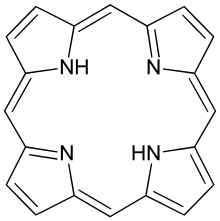
Porphyrins (/ˈpɔːrfərɪns/ POR-fər-ins) are a group of heterocyclic, macrocyclic, organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (=CH−). In vertebrates, an essential member of the porphyrin group is heme, which is a component of hemoproteins, whose functions include carrying oxygen in the bloodstream. In plants, an essential porphyrin derivative is chlorophyll, which is involved in light harvesting and electron transfer in photosynthesis.
The parent of porphyrins is porphine, a rare chemical compound of exclusively theoretical interest. Substituted porphines are called porphyrins.[1] With a total of 26 π-electrons, of which 18 π-electrons form a planar, continuous cycle, the porphyrin ring structure is often described as aromatic.[2][3] One result of the large conjugated system is that porphyrins typically absorb strongly in the visible region of the electromagnetic spectrum, i.e. they are deeply colored. The name "porphyrin" derives from Greek πορφύρα (porphyra) 'purple'.[4]
- ^ Rayati S, Malekmohammadi S (2016). "Catalytic activity of multi-wall carbon nanotube supported manganese (III) porphyrin: an efficient, selective and reusable catalyst for oxidation of alkenes and alkanes with urea–hydrogen peroxide". Journal of Experimental Nanoscience. 11 (11): 872. Bibcode:2016JENan..11..872R. doi:10.1080/17458080.2016.1179802.
- ^ Ivanov AS, Boldyrev AI (August 2014). "Deciphering aromaticity in porphyrinoids via adaptive natural density partitioning". Organic & Biomolecular Chemistry. 12 (32): 6145–6150. doi:10.1039/C4OB01018C. PMID 25002069.
- ^ Lash TD (2011). "Origin of aromatic character in porphyrinoid systems". Journal of Porphyrins and Phthalocyanines. 15 (11n12): 1093–1115. doi:10.1142/S1088424611004063.
- ^ Harper D, Buglione DC. "porphyria (n.)". The Online Etymology Dictionary. Retrieved 14 September 2014.