| |
| |

| |
| Names | |
|---|---|
| IUPAC name
Potassium carbonate
| |
| Other names
Carbonate of potash, dipotassium carbonate, sub-carbonate of potash, pearl ash, potash, salt of tartar, salt of wormwood.
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.665 |
| E number | E501(i) (acidity regulators, ...) |
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
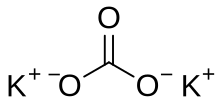
| K 2CO 3 | |
| Molar mass | 138.205 g/mol |
| Appearance | White, hygroscopic solid |
| Density | 2.43 g/cm3 |
| Melting point | 891 °C (1,636 °F; 1,164 K) |
| Boiling point | Decomposes |
| 110.3 g/100 mL (20 °C) 149.2 g/100 mL (100 °C) | |
| Solubility | |
| Acidity (pKa) | 10.25 |
| −59.0·10−6 cm3/mol | |
| Thermochemistry[1] | |
Heat capacity (C)
|
114.4 J·mol−1·K−1 |
Std molar
entropy (S⦵298) |
155.5 J·mol−1·K−1 |
Std enthalpy of
formation (ΔfH⦵298) |
−1151.0 kJ·mol−1 |
Gibbs free energy (ΔfG⦵)
|
−1063.5 kJ·mol−1 |
Enthalpy of fusion (ΔfH⦵fus)
|
27.6 kJ·mol−1 |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H302, H315, H319, H335 | |
| P261, P305+P351+P338 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
1870 mg/kg (oral, rat)[2] |
| Safety data sheet (SDS) | ICSC 1588 |
| Related compounds | |
Other anions
|
Potassium bicarbonate |
Other cations
|
Lithium carbonate Sodium carbonate Rubidium carbonate Caesium carbonate |
Related compounds
|
Ammonium carbonate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Potassium carbonate is the inorganic compound with the formula K2CO3. It is a white salt, which is soluble in water and forms a strongly alkaline solution. It is deliquescent, often appearing as a damp or wet solid. Potassium carbonate is mainly used in the production of soap and glass.[3] Commonly, it can be found as the result of leakage of alkaline batteries.[4]
- ^ CRC handbook of chemistry and physics: a ready-reference book of chemical and physical data. William M. Haynes, David R. Lide, Thomas J. Bruno (2016-2017, 97th ed.). Boca Raton, Florida. 2016. ISBN 978-1-4987-5428-6. OCLC 930681942.
{{cite book}}: CS1 maint: location missing publisher (link) CS1 maint: others (link) - ^ Chambers, Michael. "ChemIDplus - 584-08-7 - BWHMMNNQKKPAPP-UHFFFAOYSA-L - Potassium carbonate [USP] - Similar structures search, synonyms, formulas, resource links, and other chemical information". chem.sis.nlm.nih.gov. Archived from the original on 2014-08-12.
- ^ H. Schultz; G. Bauer; E. Schachl; F. Hagedorn; P. Schmittinger (2005). "Potassium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_039. ISBN 3-527-30673-0.
- ^ List, Jenny (October 19, 2022). "Crusty Leaking Cells Kill Your Tech. Just What's Going On?". Hackaday. Archived from the original on May 30, 2023.
