 Arcanite
| |
| |
| Names | |
|---|---|
| Other names
Potassium sulphate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.029.013 |
| EC Number |
|
| E number | E515(i) (acidity regulators, ...) |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
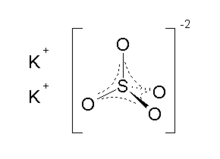
| K2SO4 | |
| Molar mass | 174.259 g/mol |
| Appearance | White solid |
| Odor | odorless |
| Density | 2.66 g/cm3[1] |
| Melting point | 1,069[2] °C (1,956 °F; 1,342 K) |
| Boiling point | 1,689 °C (3,072 °F; 1,962 K) |
| 111 g/L (20 °C) 120 g/L (25 °C) 240 g/L (100 °C) | |
Solubility product (Ksp)
|
1.32 (120 g/L) |
| Solubility | slightly soluble in glycerol insoluble in acetone, alcohol, CS2 |
| −67.0·10−6 cm3/mol | |
Refractive index (nD)
|
1.495 |
| Structure | |
| orthorhombic | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Irritant |
| GHS labelling: | |

| |
| Warning | |
| H318 | |
| P280, P305+P351+P338, P310 | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
6600 mg/kg (oral, rat)[3] |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Other anions
|
Potassium selenate Potassium tellurate |
Other cations
|
Lithium sulfate Sodium sulfate Rubidium sulfate Caesium sulfate |
Related compounds
|
Potassium hydrogen sulfate Potassium sulfite Potassium bisulfite Potassium persulfate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Potassium sulfate (US) or potassium sulphate (UK), also called sulphate of potash (SOP), arcanite, or archaically potash of sulfur, is the inorganic compound with formula K2SO4, a white water-soluble solid. It is commonly used in fertilizers, providing both potassium and sulfur.
- ^ Patnaik, Pradyot (2002). Handbook of Inorganic Chemicals. McGraw-Hill. ISBN 978-0-07-049439-8.
- ^ Windholtz, M; Budavari, S, eds. (1983). The Merck Index. Rahway, New Jersey: Merck & Co.
- ^ Chambers, Michael. "Potassium sulfate RN: 7778-80-5". ChemIDplus. United States National Library of Medicine.