 Potassium cations, K+ Superoxide anions, O−2 | |

| |
| Names | |
|---|---|
| IUPAC name
Potassium superoxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.031.574 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UN number | 2466 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| KO2 | |
| Molar mass | 71.096 g·mol−1 |
| Appearance | yellow solid |
| Density | 2.14 g/cm3, solid |
| Melting point | 560 °C (1,040 °F; 833 K) (decomposes) |
| Hydrolysis | |
| +3230·10−6 cm3/mol[1] | |
| Structure | |
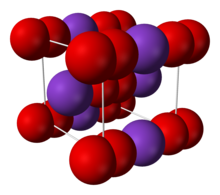
| Body-centered tetragonal[2][3] | |
| Thermochemistry | |
Std molar
entropy (S⦵298) |
117 J/(mol·K)[4] |
Std enthalpy of
formation (ΔfH⦵298) |
−283 kJ/mol[4] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Corrosive, oxidizer, reacts violently with water |
| GHS labelling:[5] | |
 
| |
| Danger | |
| H271, H314 | |
| P210, P220, P221, P260, P264, P280, P283, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P306+P360, P310, P321, P363, P370+P378, P371+P380+P375, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other cations
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Potassium superoxide is an inorganic compound with the formula KO2.[6] It is a yellow paramagnetic solid that decomposes in moist air. It is a rare example of a stable salt of the superoxide anion. It is used as a CO2 scrubber, H2O dehumidifier, and O2 generator in rebreathers, spacecraft, submarines, and spacesuits.
- ^ "Handbook of Chemistry and Physics 102nd Edition". CRC Press.
- ^ Cite error: The named reference
Abrahamswas invoked but never defined (see the help page). - ^ "Information card for entry 2310803". Crystallography Open Database. Retrieved 28 July 2022.
- ^ a b Zumdahl, Steven S. (2009). Chemical Principles (6th ed.). Houghton Mifflin. p. A22. ISBN 978-0-618-94690-7.
- ^ "Potassium superoxide". pubchem.ncbi.nlm.nih.gov. Retrieved 14 December 2021.
- ^ Hayyan M.; Hashim M. A.; AlNashef I. M. (2016). "Superoxide Ion: Generation and Chemical Implications". Chem. Rev. 116 (5): 3029–3085. doi:10.1021/acs.chemrev.5b00407. PMID 26875845.
