 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Effient, Efient |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a609027 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ≥79% |
| Protein binding | Active metabolite: ~98% |
| Elimination half-life | ~7 h (range 2 h to 15 h) |
| Excretion | Urine (~68% inactive metabolites); feces (27% inactive metabolites) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.228.719 |
| Chemical and physical data | |
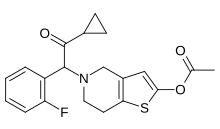
| Formula | C20H20FNO3S |
| Molar mass | 373.44 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Prasugrel, sold under the brand names Effient and Efient, is a medication used to prevent formation of blood clots. It is a platelet inhibitor and an irreversible antagonist of P2Y12 ADP receptors and is of the thienopyridine drug class. It was developed by Daiichi Sankyo Co. and produced by Ube and marketed in the United States in cooperation with Eli Lilly and Company.
Prasugrel was approved for use in the European Union in February 2009,[2] and in the US in July 2009, for the reduction of thrombotic cardiovascular events (including stent thrombosis) in people with acute coronary syndrome (ACS) who are to be managed with percutaneous coronary intervention (PCI).[3]
- ^ "Efient EPAR". European Medicines Agency (EMA). 25 February 2009. Retrieved 5 September 2024.
- ^ "European Public Assessment Report for Efient" (PDF). EMA. 2009.
- ^ Baker WL, White CM (2009). "Role of prasugrel, a novel P2Y(12) receptor antagonist, in the management of acute coronary syndromes". American Journal of Cardiovascular Drugs. 9 (4): 213–229. doi:10.2165/1131209-000000000-00000. PMID 19655817. S2CID 37160513.