 | |
 | |
| Clinical data | |
|---|---|
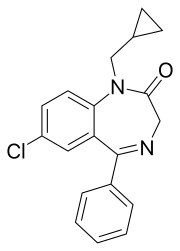
| Other names | 9-chloro-2-(cyclopropylmethyl)-6-phenyl-2,5-diazabicyclo[5.4.0]undeca-5,8,10,12-tetraen- 3-one |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a601036 |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Elimination half-life | 36–200 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.019.069 |
| Chemical and physical data | |
| Formula | C19H17ClN2O |
| Molar mass | 324.81 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Prazepam is a benzodiazepine derivative drug developed by Warner-Lambert in the 1960s.[2] It possesses anxiolytic, anticonvulsant, sedative and skeletal muscle relaxant properties.[3] Prazepam is a prodrug for desmethyldiazepam which is responsible for the therapeutic effects of prazepam.[4]
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ US Patent 3192199 – Process for the production of I-CYCLO- ALKYL derivatives of I,X-BENZODIAZEPINE
- ^ Shader RI, Greenblatt DJ (1979). "Benzodiazepines: some aspects of their clinical pharmacology". Ciba Foundation Symposium. Novartis Foundation Symposia. 1979 (74): 141–155. doi:10.1002/9780470720578.ch9. ISBN 9780470720578. PMID 45081.
- ^ Jacqmin P, Ansseau M (1988). "Comparison of sublingual and oral prazepam in normal subjects. II. Pharmacokinetic and pharmacodynamic data". Neuropsychobiology. 19 (4): 186–191. doi:10.1159/000118458. PMID 2854609.