 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | Parenteral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | N/A |
| Metabolism | Hydrolysis by plasma esterases |
| Elimination half-life | 40–84 seconds |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.388 |
| Chemical and physical data | |
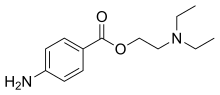
| Formula | C13H20N2O2 |
| Molar mass | 236.315 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Procaine is a local anesthetic drug of the amino ester group. It is most commonly used in dental procedures to numb the area around a tooth[1] and is also used to reduce the pain of intramuscular injection of penicillin. Owing to the ubiquity of the trade name Novocain or Novocaine, in some regions, procaine is referred to generically as novocaine. It acts mainly as a sodium channel blocker.[2] Today, it is used therapeutically in some countries due to its sympatholytic, anti-inflammatory, perfusion-enhancing, and mood-enhancing effects.[3]
Procaine was first synthesized in 1905,[4] shortly after amylocaine.[5] It was created by the chemist Alfred Einhorn who gave the chemical the trade name Novocaine, from the Latin nov- (meaning "new") and -caine, a common ending for alkaloids used as anesthetics. It was introduced into medical use by surgeon Heinrich Braun.
Prior to the discovery of amylocaine and procaine, cocaine was a commonly used local anesthetic.[6] Einhorn wished his new discovery to be used for amputations, but for this surgeons preferred general anesthesia. Dentists, however, found it very useful.[7]
- ^ "How long does numbness last after the dentist?". Medical News Today. May 22, 2018. Retrieved July 14, 2020.
- ^ "Procaine (DB00721)". DrugBank. 2009-06-23.
- ^ Hahn-Godeffroy JD (2011). "Procain-Reset: Ein Therapiekonzept zur Behandlung chronischer Erkrankungen" [Procaine reset: A therapy concept for the treatment of chronic diseases.]. Schweizerische Zeitschrift für Ganzheitsmedizin [Swiss Journal of Integrative Medicine] (in German). 23 (5): 291–6. doi:10.1159/000332021.
- ^ Ritchie JM, Greene NM (1990). "Local Anesthetics". In Gilman AG, Rall TW, Nies AS, Taylor P (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics (8th ed.). New York: Pergamon Press. p. 311. ISBN 0-08-040296-8.
- ^ Minard R (18 October 2006). "The Preparation of the Local Anesthetic, Benzocaine, by an Esterification Reaction" (PDF). Archived from the original (PDF) on 20 July 2011. Retrieved 10 March 2011.
Adapted from Introduction to Organic Laboratory Techniques: A Microscale Approach, Pavia, Lampman, Kriz & Engel, 1989.
- ^ Ruetsch YA, Böni T, Borgeat A (August 2001). "From cocaine to ropivacaine: the history of local anesthetic drugs". Current Topics in Medicinal Chemistry. 1 (3): 175–82. doi:10.2174/1568026013395335. PMID 11895133.
- ^ Drucker P (May 1985). "The discipline of innovation". Harvard Business Review. 3 (3): 68. PMID 10272260.