| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 1696878 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.693 | ||
| EC Number |
| ||
| 852 | |||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1077 In Liquefied petroleum gas: 1075 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C3H6 | |||
| Molar mass | 42.081 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Density | 1.81 kg/m3, gas (1.013 bar, 15 °C) 1.745 kg/m3, gas (1.013 bar, 25 °C) 613.9 kg/m3, liquid | ||
| Melting point | −185.2 °C (−301.4 °F; 88.0 K) | ||
| Boiling point | −47.6 °C (−53.7 °F; 225.6 K) | ||
| 0.61 g/m3 | |||
| -31.5·10−6 cm3/mol | |||
| Viscosity | 8.34 µPa·s at 16.7 °C | ||
| Structure | |||
| 0.366 D (gas) | |||
| Hazards | |||
| GHS labelling:[3] | |||

| |||
| Danger | |||
| H220 | |||
| P210, P377, P381, P403 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −108 °C (−162 °F; 165 K) | ||
| Safety data sheet (SDS) | External MSDS | ||
| Related compounds | |||
Related alkenes;
related groups |
Ethylene, Isomers of Butylene; Allyl, Propenyl | ||
Related compounds
|
Propane, Propyne Propadiene, 1-Propanol 2-Propanol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
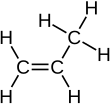
Propylene, also known as propene, is an unsaturated organic compound with the chemical formula CH3CH=CH2. It has one double bond, and is the second simplest member of the alkene class of hydrocarbons. It is a colorless gas with a faint petroleum-like odor.[4]
Propylene is a product of combustion from forest fires, cigarette smoke, and motor vehicle and aircraft exhaust.[5] It was discovered in 1850 by A. W. von Hoffman's student Captain (later Major General[6]) John Williams Reynolds as the only gaseous product of thermal decomposition of amyl alcohol to react with chlorine and bromine.[7]
- ^ "General Principles, Rules, and Conventions". Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 31. doi:10.1039/9781849733069-00001. ISBN 978-0-85404-182-4.
- ^ Moss, G.P. (web version). "P-14.3 Locants". Nomenclature of Organic Chemistry. IUPAC Recommendations and Preferred Names 2013. London: Queen Mary University. Section P-14.3.4.2 (d). Retrieved 23 August 2024.
- ^ "Propylene". pubchem.ncbi.nlm.nih.gov. Retrieved 14 December 2021.
- ^ "Propylene".
- ^ Morgott, David (2018-01-04). "The Human Exposure Potential from Propylene Releases to the Environment". International Journal of Environmental Research and Public Health. 15 (1): 66. doi:10.3390/ijerph15010066. ISSN 1660-4601. PMC 5800165. PMID 29300328.
- ^ "Maj Gen John Williams Reynolds, FCS". geni_family_tree. 1816-12-25. Retrieved 2023-12-30.
- ^ Rasmussen, Seth C. (2018), Rasmussen, Seth C. (ed.), "Introduction", Acetylene and Its Polymers: 150+ Years of History, SpringerBriefs in Molecular Science, Cham: Springer International Publishing, pp. 1–19, doi:10.1007/978-3-319-95489-9_1, ISBN 978-3-319-95489-9, retrieved 2023-12-30