| |||

| |||
| Names | |||
|---|---|---|---|
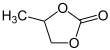
| Preferred IUPAC name
4-Methyl-1,3-dioxolan-2-one | |||
| Other names
(RS)-4-Methyl-1,3-dioxolan-2-one
Cyclic propylene carbonate Carbonic acid propylene ester Cyclic 1,2-propylene carbonate Propylene glycol cyclic carbonate 1,2-Propanediol carbonate 4-Methyl-2-oxo-1,3-dioxolane Arconate 5000 Texacar PC | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.003.248 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C4H6O3 | |||
| Molar mass | 102.089 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 1.205 g/cm3 | ||
| Melting point | −48.8 °C (−55.8 °F; 224.3 K) | ||
| Boiling point | 242 °C (468 °F; 515 K) | ||
| Very soluble (240 g/L at 20°C) | |||
Refractive index (nD)
|
1.4189 | ||
| Structure | |||
| 4.9 D | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Irritant | ||
| GHS labelling:[3] | |||

| |||
| Warning | |||
| H319 | |||
| P305+P351+P338 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 132 °C (270 °F; 405 K) | ||
| 455 °C (851 °F; 728 K) | |||
| Safety data sheet (SDS) | MSDS by Mallinckrodt Baker | ||
| Related compounds | |||
Related compounds
|
Ethylene carbonate Dimethyl carbonate | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Propylene carbonate (often abbreviated PC) is an organic compound with the formula C4H6O3. It is a cyclic carbonate ester derived from propylene glycol.[4] This colorless and odorless liquid is useful as a polar, aprotic solvent.[5] Propylene carbonate is chiral, but is used as the racemic mixture in most contexts.
- ^ Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. ISBN 1-4398-5511-0.
- ^ Propylene carbonate at Sigma-Aldrich.
- ^ GHS: GESTIS 070730
- ^ WebBook page for propylene carbonate.
- ^ Cite error: The named reference
ullmannwas invoked but never defined (see the help page).