| |

| |
| Names | |
|---|---|
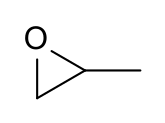
| Preferred IUPAC name
(2R)-2-Methyloxirane (2S)-2-Methyloxirane | |
| Other names
Propylene oxide
Epoxypropane Propylene epoxide 1,2-Propylene oxide Methyl oxirane 1,2-Epoxypropane Propene oxide Methyl ethylene oxide Methylethylene oxide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.000.800 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H6O | |
| Molar mass | 58.080 g·mol−1 |
| Appearance | Colourless liquid |
| Odor | benzene-like[1] |
| Density | 0.859 g/cm3[2] |
| Melting point | −111.9 °C (−169.4 °F; 161.2 K)[2] |
| Boiling point | 35 °C (95 °F; 308 K)[2] |
| 41% (20 °C)[1] | |
| Vapor pressure | 445 mmHg (20 °C)[1] |
| −4.25×10−5 cm3/mol[3] | |
Refractive index (nD)
|
1.3660[2] |
| Thermochemistry | |
Heat capacity (C)
|
120.4 J·(K·mol)−1 |
Std molar
entropy (S⦵298) |
196.5 J·(K·mol)−1 |
Std enthalpy of
formation (ΔfH⦵298) |
−123.0 kJ·mol−1[4] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Extremely flammable[5][6] |
| GHS labelling: | |
  
| |
| Danger | |
| NFPA 704 (fire diamond) | |
| Flash point | −37 °C (−35 °F; 236 K) |
| 747 °C (1,377 °F; 1,020 K) | |
| Explosive limits | 2.3–36%[1] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
660 mg/kg (guinea pig, oral) 380 mg/kg (rat, oral) 440 mg/kg (mouse, oral) 1140 mg/kg (rat, oral) 690 mg/kg (guinea pig, oral)[7] |
LC50 (median concentration)
|
1740 ppm (mouse, 4 h) 4000 ppm (rat, 4 h)[7] |
LCLo (lowest published)
|
2005 ppm (dog, 4 h) 4000 ppm (guinea pig, 4 h)[7] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 100 ppm (240 mg/m3)[1] |
REL (Recommended)
|
Ca[1] |
IDLH (Immediate danger)
|
Ca [400 ppm][1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Propylene oxide is an acutely toxic and carcinogenic organic compound with the molecular formula C3H6O. This colourless volatile liquid with an odour similar to ether, is produced on a large scale industrially. Its major application is its use for the production of polyether polyols for use in making polyurethane plastics. It is a chiral epoxide, although it is commonly used as a racemic mixture.
This compound is sometimes called 1,2-propylene oxide to distinguish it from its isomer 1,3-propylene oxide, better known as oxetane.
- ^ a b c d e f g NIOSH Pocket Guide to Chemical Hazards. "#0538". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b c d Haynes 2011, p. 3.384
- ^ Haynes 2011, p. 3.577
- ^ Haynes 2011, p. 5.24
- ^ "NFPA DIAMOND". www.otrain.com.
- ^ GOV, NOAA Office of Response and Restoration, US. "PROPYLENE OXIDE | CAMEO Chemicals | NOAA". cameochemicals.noaa.gov.
- ^ a b c "Propylene oxide". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
