A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.[1]
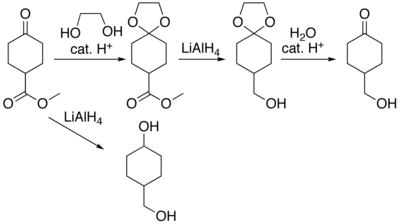
In many preparations of delicate organic compounds, specific parts of the molecules cannot survive the required reagents or chemical environments. These parts (functional groups) must be protected. For example, lithium aluminium hydride is a highly reactive reagent that usefully reduces esters to alcohols. It always reacts with carbonyl groups, and cannot be discouraged by any means. When an ester must be reduced in the presence of a carbonyl, hydride attack on the carbonyl must be prevented. One way to do so converts the carbonyl into an acetal, which does not react with hydrides. The acetal is then called a protecting group for the carbonyl. After the hydride step is complete, aqueous acid removes the acetal, restoring the carbonyl. This step is called deprotection.
Protecting groups are more common in small-scale laboratory work and initial development than in industrial production because they add additional steps and material costs. However, compounds with repetitive functional groups – generally, biomolecules like peptides, oligosaccharides or nucleotides – may require protecting groups to order their assembly. Also, cheap chiral protecting groups may often shorten an enantioselective synthesis (e.g. shikimic acid for oseltamivir).
As a rule, the introduction of a protecting group is straightforward. The difficulties honestly lie in their stability and in selective removal. Apparent problems in synthesis strategies with protecting groups are rarely documented in the academic literature.[2]
- ^ Theodora W. Greene; Peter G. M. Wuts (1999). Protecting Groups in Organic Synthesis (3 ed.). J. Wiley. ISBN 978-0-471-16019-9.
- ^ Michael Schelhaas, Herbert Waldmann: "Schutzgruppenstrategien in der organischen Synthese", in: Angewandte Chemie, 1996, 103, pp. 2194; doi:10.1002/ange.19961081805 (in German).