 | |
| Clinical data | |
|---|---|
| Trade names | Vivactyl, others |
| Other names | Amimethyline; Protriptyline hydrochloride; MK-240 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604025 |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 77–93%[2] |
| Protein binding | 92%[2] |
| Metabolism | Hepatic |
| Elimination half-life | 54–92 hours |
| Excretion | Urine: 50%[2] Feces: minor[2] |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.006.474 |
| Chemical and physical data | |
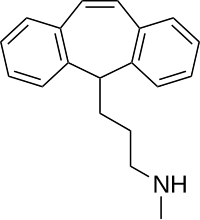
| Formula | C19H21N |
| Molar mass | 263.384 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Protriptyline, sold under the brand name Vivactil among others, is a tricyclic antidepressant (TCA), specifically a secondary amine, indicated for the treatment of depression and attention-deficit hyperactivity disorder (ADHD). Uniquely among most of the TCAs, protriptyline tends to be energizing instead of sedating, and is sometimes used for narcolepsy to achieve a wakefulness-promoting effect.
TCAs including protriptyline are also used to reduce the incidence of recurring headaches such as migraine, and for other types of chronic pain.
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ a b c d Lemke TL, Williams DA (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 588–. ISBN 978-1-60913-345-0.