| |
| Names | |
|---|---|
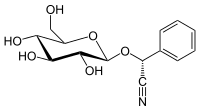
| IUPAC name
(R)-(β-D-Glucopyranosyloxy)(phenyl)acetonitrile
| |
| Systematic IUPAC name
(R)-Phenyl{[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}acetonitrile | |
| Other names
(R)-Prunasin
D-Prunasin D-Mandelonitrile-β-D-glucoside Prulaurasin Laurocerasin Sambunigrin | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.002.489 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C14H17NO6 | |
| Molar mass | 295.291 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
(R)-prunasin is a cyanogenic glycoside related to amygdalin. Chemically, it is the glucoside of (R)-mandelonitrile.