| |
| Names | |
|---|---|
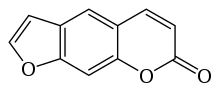
| Preferred IUPAC name
7H-Furo[3,2-g][1]benzopyran-7-one | |
| Identifiers | |
3D model (JSmol)
|
|
| 152784 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.581 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C11H6O3 | |
| Molar mass | 186.16 g/mol |
| Melting point | 158 to 161 °C (316 to 322 °F; 431 to 434 K) |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H302, H315, H319, H335 | |
| P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Psoralen (also called psoralene) is the parent compound in a family of naturally occurring organic compounds known as the linear furanocoumarins. It is structurally related to coumarin by the addition of a fused furan ring, and may be considered as a derivative of umbelliferone. Psoralen occurs naturally in the seeds of Psoralea corylifolia, as well as in the common fig, celery, parsley, West Indian satinwood, and in all citrus fruits. It is widely used in PUVA (psoralen + UVA) treatment for psoriasis, eczema, vitiligo, and cutaneous T-cell lymphoma; these applications are typically through the use of medications such as Methoxsalen. Many furanocoumarins are extremely toxic to fish, and some are deposited in streams in Indonesia to catch fish.[1]
- ^ Dean, F. M. (1963). Naturally occurring oxygen ring compounds. London: Butterworths.