 | |
| Names | |
|---|---|
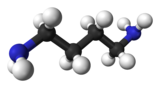
| Preferred IUPAC name
Butane-1,4-diamine | |
| Other names
1,4-Diaminobutane, 1,4-Butanediamine
| |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| 605282 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.440 |
| EC Number |
|
| 1715 | |
| KEGG | |
| MeSH | Putrescine |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2928 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H12N2 | |
| Molar mass | 88.154 g·mol−1 |
| Appearance | Colourless crystals |
| Odor | fishy-ammoniacal, pungent |
| Density | 0.877 g/mL |
| Melting point | 27.5 °C (81.5 °F; 300.6 K) |
| Boiling point | 158.6 °C; 317.4 °F; 431.7 K |
| Miscible | |
| log P | −0.466 |
| Vapor pressure | 2.33 mm Hg at 25 deg C (est) |
Henry's law
constant (kH) |
3.54x10−10 atm-cu m/mol at 25 deg C (est) |
Refractive index (nD)
|
1.457 |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H228, H302, H312, H314, H331 | |
| P210, P261, P280, P305+P351+P338, P310 | |
| Flash point | 51 °C (124 °F; 324 K) |
| Explosive limits | 0.98–9.08% |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
|
| Related compounds | |
Related alkanamines
|
|
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Putrescine is an organic compound with the formula (CH2)4(NH2)2. It is a colorless solid that melts near room temperature. It is classified as a diamine.[3] Together with cadaverine, it is largely responsible for the foul odor of putrefying flesh, but also contributes to other unpleasant odors.
- ^ Thalladi, V.R.; Boese, R.; Weiss, H.-C. (2001). "CSD Entry: QATWAJ : 1,4-Butanediamine". Cambridge Structural Database: Access Structures. Cambridge Crystallographic Data Centre. doi:10.5517/cc4g850. Retrieved 2021-11-07.
- ^ Thalladi, V. R.; Boese, R.; Weiss, H.-C. (2000). "The Melting Point Alternation in α,ω-Alkanediols and α,ω-Alkanediamines: Interplay between Hydrogen Bonding and Hydrophobic Interactions". Angew. Chem. Int. Ed. 39 (5): 918–922. doi:10.1002/(SICI)1521-3773(20000303)39:5<918::AID-ANIE918>3.0.CO;2-E. PMID 10760893.
- ^ Eller, Karsten; Henkes, Erhard; Rossbacher, Roland; Höke, Hartmut (2000). "Amines, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_001. ISBN 3527306730.