| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Pyridinium chlorochromate
| |||
| Other names
PCC; Corey-Suggs reagent
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.043.253 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C5H6ClCrNO3 | |||
| Molar mass | 215.56 g/mol | ||
| Appearance | yellow-orange solid[1] | ||
| Melting point | 205 °C (401 °F; 478 K) | ||
| Solubility in other solvents | soluble in acetone, acetonitrile, THF | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Toxic, oxidizer, carcinogenic, strong environmental pollutant | ||
| GHS labelling: | |||
   
| |||
| Danger | |||
| H272, H317, H350, H410 | |||
| P201, P221, P273, P280, P302+P352, P308+P313 | |||
| NFPA 704 (fire diamond) | |||
| Safety data sheet (SDS) | external SDS | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||

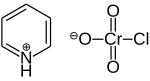
Pyridinium chlorochromate (PCC) is a yellow-orange salt with the formula [C5H5NH]+[CrO3Cl]−. It is a reagent in organic synthesis used primarily for oxidation of alcohols to form carbonyls. A variety of related compounds are known with similar reactivity. PCC offers the advantage of the selective oxidation of alcohols to aldehydes or ketones, whereas many other reagents are less selective.[1]
- ^ a b Piancatelli, G.; Luzzio, F. A. (2007). "Pyridinium Chlorochromate". e-EROS Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. doi:10.1002/9780470842898.rp288.pub2. ISBN 978-0471936237.
- ^ "Safety Data Sheet". Acros Organics. 2015. Retrieved 2016-06-10.