| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Pyrrolidine[1] | |||
| Other names
Azolidine
Azacyclopentane Tetrahydropyrrole Prolamine Azolane | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 102395 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.227 | ||
| EC Number |
| ||
| 1704 | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1922 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C4H9N | |||
| Molar mass | 71.123 g·mol−1 | ||
| Appearance | Clear colorless liquid | ||
| Density | 0.866 g/cm3 | ||
| Melting point | −63 °C (−81 °F; 210 K) | ||
| Boiling point | 87 °C (189 °F; 360 K) | ||
| Miscible | |||
| Acidity (pKa) | 11.27 (pKa of conjugate acid in water),[2] 19.56 (pKa of conjugate acid in acetonitrile)[3] | ||
| -54.8·10−6 cm3/mol | |||
Refractive index (nD)
|
1.4402 at 28°C | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
highly flammable, harmful, corrosive, possible mutagen | ||
| GHS labelling: | |||
  
| |||
| Danger | |||
| H225, H302, H314, H332 | |||
| P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P280, P301+P312, P301+P330+P331, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P310, P312, P321, P330, P363, P370+P378, P403+P235, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 3 °C (37 °F; 276 K) | ||
| 345 °C (653 °F; 618 K) | |||
| Safety data sheet (SDS) | MSDS | ||
| Related compounds | |||
Related nitrogen heterocyclic compounds
|
Pyrrole (aromatic with two double bonds) Pyrroline (one double bond) Pyrrolizidine (two pentagonal rings) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
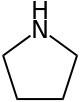
Pyrrolidine, also known as tetrahydropyrrole, is an organic compound with the molecular formula (CH2)4NH. It is a cyclic secondary amine, also classified as a saturated heterocycle. It is a colourless liquid that is miscible with water and most organic solvents. It has a characteristic odor that has been described as "ammoniacal, fishy, shellfish-like".[4] In addition to pyrrolidine itself, many substituted pyrrolidines are known.
- ^ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 142. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ^ Hall, H. K. (1957). "Correlation of the Base Strengths of Amines". Journal of the American Chemical Society. 79 (20): 5441–5444. doi:10.1021/ja01577a030.
- ^ Kaljurand, I.; Kütt, A.; Sooväli, L.; Rodima, T.; Mäemets, V.; Leito, I.; Koppel, I. A. (2005). "Extension of the Self-Consistent Spectrophotometric Basicity Scale in Acetonitrile to a Full Span of 28 pKa Units: Unification of Different Basicity Scales". The Journal of Organic Chemistry. 70 (3): 1019–1028. doi:10.1021/jo048252w. PMID 15675863.
- ^ Pyrrolidine Archived 2017-11-21 at the Wayback Machine, The Good Scents Company