| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Quinoline[2] | |||
Systematic IUPAC name
| |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| 107477 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.865 | ||
| EC Number |
| ||
| 27201 | |||
| KEGG | |||
| MeSH | Quinolines | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2656 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C9H7N | |||
| Molar mass | 129.16 g/mol | ||
| Appearance | Colorless oily liquid | ||
| Density | 1.093 g/mL | ||
| Melting point | −15 °C (5 °F; 258 K) | ||
| Boiling point | 237 °C (459 °F; 510 K) , 760 mm Hg; 108–110 °C (226–230 °F), 11 mm Hg | ||
| Slightly soluble | |||
| Solubility | Soluble in alcohol, ether, and carbon disulfide | ||
| Acidity (pKa) | 4.85 (conjugated acid)[3] | ||
| −86.0·10−6 cm3/mol | |||
| Thermochemistry | |||
Std enthalpy of
formation (ΔfH⦵298) |
174.9 kJ·mol−1 | ||
| Hazards | |||
| GHS labelling: | |||
  
| |||
| Danger | |||
| H302, H312, H315, H319, H341, H350, H411 | |||
| P201, P202, P264, P270, P273, P280, P281, P301+P312, P302+P352, P305+P351+P338, P308+P313, P312, P321, P322, P330, P332+P313, P337+P313, P362, P363, P391, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 101 °C (214 °F; 374 K) | ||
| 400 °C (752 °F; 673 K) | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
331 mg/kg | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
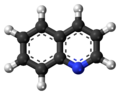
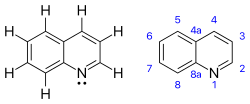
Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only slightly soluble in cold water but dissolves readily in hot water and most organic solvents.[4] Quinoline itself has few applications, but many of its derivatives are useful in diverse applications. A prominent example is quinine, an alkaloid found in plants. Over 200 biologically active quinoline and quinazoline alkaloids are identified.[5][6] 4-Hydroxy-2-alkylquinolines (HAQs) are involved in antibiotic resistance.
- ^ "QUINOLINE (BENZOPYRIDINE)". Chemicalland21.com. Retrieved 2012-06-14.
- ^ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. pp. 4, 211. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
The name 'quinoline' is a retained name that is preferred to the alternative systematic fusion names '1-benzopyridine' or 'benzo[b]pyridine'.
- ^ Brown, H.C., et al., in Baude, E.A. and Nachod, F.C., Determination of Organic Structures by Physical Methods, Academic Press, New York, 1955.
- ^ Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica. Vol. 22 (11th ed.). Cambridge University Press. p. 759.
- ^ Shang, XF; Morris-Natschke, SL; Liu, YQ; Guo, X; Xu, XS; Goto, M; Li, JC; Yang, GZ; Lee, KH (May 2018). "Biologically active quinoline and quinazoline alkaloids part I." Medicinal Research Reviews. 38 (3): 775–828. doi:10.1002/med.21466. PMC 6421866. PMID 28902434.
- ^ Shang, Xiao-Fei; Morris-Natschke, Susan L.; Yang, Guan-Zhou; Liu, Ying-Qian; Guo, Xiao; Xu, Xiao-Shan; Goto, Masuo; Li, Jun-Cai; Zhang, Ji-Yu; Lee, Kuo-Hsiung (September 2018). "Biologically active quinoline and quinazoline alkaloids part II". Medicinal Research Reviews. 38 (5): 1614–1660. doi:10.1002/med.21492. PMC 6105521. PMID 29485730.