Radioactivity is generally used in life sciences for highly sensitive and direct measurements of biological phenomena, and for visualizing the location of biomolecules radiolabelled with a radioisotope.
All atoms exist as stable or unstable isotopes and the latter decay at a given half-life ranging from attoseconds to billions of years; radioisotopes useful to biological and experimental systems have half-lives ranging from minutes to months. In the case of the hydrogen isotope tritium (half-life = 12.3 years) and carbon-14 (half-life = 5,730 years), these isotopes derive their importance from all organic life containing hydrogen and carbon and therefore can be used to study countless living processes, reactions, and phenomena. Most short lived isotopes are produced in cyclotrons, linear particle accelerators, or nuclear reactors and their relatively short half-lives give them high maximum theoretical specific activities which is useful for detection in biological systems.

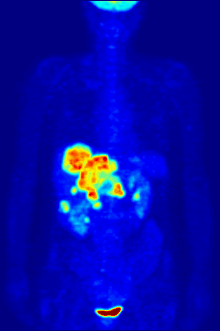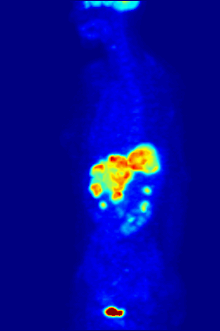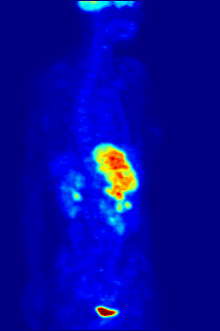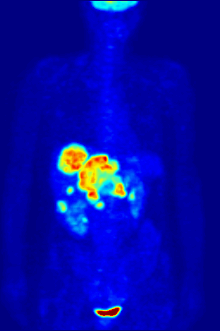
Radiolabeling is a technique used to track the passage of a molecule that incorporates a radioisotope through a reaction, metabolic pathway, cell, tissue, organism, or biological system. The reactant is 'labeled' by replacing specific atoms by their isotope. Replacing an atom with its own radioisotope is an intrinsic label that does not alter the structure of the molecule. Alternatively, molecules can be radiolabeled by chemical reactions that introduce an atom, moiety, or functional group that contains a radionuclide. For example, radio-iodination of peptides and proteins with biologically useful iodine isotopes is easily done by an oxidation reaction that replaces the hydroxyl group with iodine on tyrosine and histadine residues. Another example is to use chelators such DOTA that can be chemically coupled to a protein; the chelator in turn traps radiometals thus radiolabeling the protein. This has been used for introducing Yttrium-90 onto a monoclonal antibody for therapeutic purposes and for introducing Gallium-68 onto the peptide Octreotide for diagnostic imaging by PET imaging.[1] (See DOTA uses.)
Radiolabeling is not necessary for some applications. For some purposes, soluble ionic salts can be used directly without further modification (e.g., gallium-67, gallium-68, and radioiodine isotopes). These uses rely on the chemical and biological properties of the radioisotope itself, to localize it within the organism or biological system.
Molecular imaging is the biomedical field that employs radiotracers to visualize and quantify biological processes using positron emission tomography (PET) and single-photon emission computed tomography (SPECT) imaging. Again, a key feature of using radioactivity in life science applications is that it is a quantitative technique, so PET/SPECT not only reveals where a radiolabelled molecule is but how much is there.
Radiobiology (also known as radiation biology) is a field of clinical and basic medical sciences that involves the study of the action of radioactivity on biological systems. The controlled action of deleterious radioactivity on living systems is the basis of radiation therapy.
- ^ Breeman, W. A. P.; De Blois, E.; Sze Chan, H.; Konijnenberg, M.; Kwekkeboom, D. J.; Krenning, E. P. (2011). "68Ga-labeled DOTA-Peptides and 68Ga-labeled Radiopharmaceuticals for Positron Emission Tomography: Current Status of Research, Clinical Applications, and Future Perspectives". Seminars in Nuclear Medicine. 41 (4): 314–321. doi:10.1053/j.semnuclmed.2011.02.001. PMID 21624565.