 | |
| Clinical data | |
|---|---|
| Trade names | Altace, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a692027 |
| License data | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 28% |
| Protein binding | 73% (ramipril) 56% (ramiprilat) |
| Metabolism | Liver, to ramiprilat |
| Elimination half-life | 13 to 17 hours |
| Excretion | Kidney (60%) and fecal (40%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.170.726 |
| Chemical and physical data | |
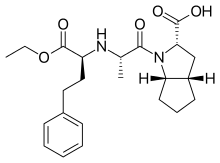
| Formula | C23H32N2O5 |
| Molar mass | 416.518 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 109 °C (228 °F) |
| |
| |
| (verify) | |
Ramipril, sold under the brand name Altace among others, is an ACE inhibitor type medication used to treat high blood pressure, heart failure, and diabetic kidney disease.[1] It can also be used as a preventative medication in patients over 55 years old to reduce the risk of having a heart attack, stroke or cardiovascular death in patients shown to be at high risk, such as some diabetics and patients with vascular disease.[2][3][4] It is a reasonable initial treatment for high blood pressure.[1] It is taken by mouth.[1]
Common side effects include headaches, dizziness, fatigue, and cough.[1] Serious side effects may include liver problems, angioedema, kidney problems, and high blood potassium.[1] Use in pregnancy and breastfeeding is not recommended.[5] It is an ACE inhibitor and works by decreasing renin-angiotensin-aldosterone system activity.[1]
Ramipril was patented in 1981 and approved for medical use in 1989.[6] It is available as a generic medication.[7] In 2022, it was the 187th most commonly prescribed medication in the United States, with more than 2 million prescriptions.[8][9]
- ^ a b c d e f "Ramipril Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 3 March 2019.
- ^ Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G (January 2000). "Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients". The New England Journal of Medicine. 342 (3): 145–53. doi:10.1056/NEJM200001203420301. PMID 10639539.
- ^ HOPE study investigators (January 2000). "Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy". The Lancet. 355 (9200): 253–259. doi:10.1016/S0140-6736(99)12323-7. S2CID 1863533.
- ^ Savarese G, Costanzo P, Cleland JG, Vassallo E, Ruggiero D, Rosano G, et al. (January 2013). "A meta-analysis reporting effects of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in patients without heart failure". Journal of the American College of Cardiology. 61 (2): 131–42. doi:10.1016/j.jacc.2012.10.011. PMID 23219304.
- ^ "Ramipril Pregnancy and Breastfeeding Warnings". Drugs.com. Retrieved 3 March 2019.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 469. ISBN 9783527607495.
- ^ British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 172–173. ISBN 9780857113382.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Ramipril Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.