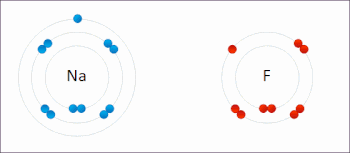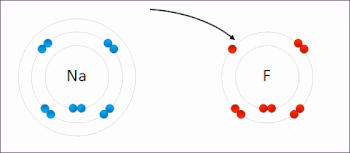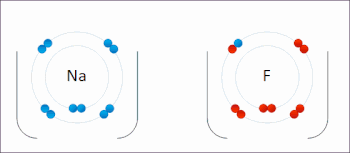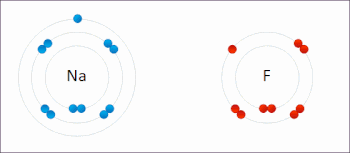
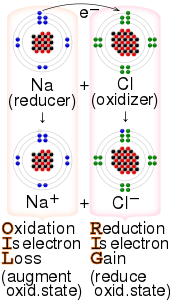
Redox (/ˈrɛdɒks/ RED-oks, /ˈriːdɒks/ REE-doks, reduction–oxidation[2] or oxidation–reduction[3]: 150 ) is a type of chemical reaction in which the oxidation states of the reactants change.[4] Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. The oxidation and reduction processes occur simultaneously in the chemical reaction.
There are two classes of redox reactions:
- Electron-transfer – Only one (usually) electron flows from the atom, ion, or molecule being oxidized to the atom, ion, or molecule that is reduced. This type of redox reaction is often discussed in terms of redox couples and electrode potentials.
- Atom transfer – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously, the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with forming oxides, other chemical species can serve the same function.[5] In hydrogenation, bonds like C=C are reduced by transfer of hydrogen atoms.
- ^ "Metals". Bitesize. BBC. Archived from the original on November 3, 2022.
- ^ "redox – definition of redox in English | Oxford Dictionaries". Oxford Dictionaries | English. Archived from the original on October 1, 2017. Retrieved May 15, 2017.
- ^ Petrucci, Ralph H.; Harwood, William S.; Herring, F. Geoffrey (2002). General Chemistry (8th ed.). Prentice-Hall. ISBN 0-13-014329-4.
- ^ "Redox Reactions". wiley.com. Archived from the original on May 30, 2012. Retrieved May 9, 2012.
- ^ Haustein, Catherine Hinga (2014). "Oxidation-reduction reaction". In K. Lee Lerner; Brenda Wilmoth Lerner (eds.). The Gale Encyclopedia of Science (5th ed.). Farmington Hills, MI: Gale Group.