| Rhomboid | |||||||||
|---|---|---|---|---|---|---|---|---|---|
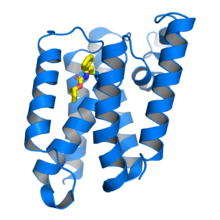 Escherichia coli rhomboid protease GlpG in complex with a beta-lactam inhibitor (yellow) bound to the catalytic serine residue. From PDB: 3ZMH.[1] | |||||||||
| Identifiers | |||||||||
| Symbol | Rhomboid | ||||||||
| Pfam | PF01694 | ||||||||
| Pfam clan | CL0207 | ||||||||
| InterPro | IPR002610 | ||||||||
| MEROPS | S54 | ||||||||
| SCOP2 | 144092 / SCOPe / SUPFAM | ||||||||
| OPM superfamily | 165 | ||||||||
| OPM protein | 2ic8 | ||||||||
| |||||||||
The rhomboid proteases are a family of enzymes that exist in almost all species. They are proteases: they cut the polypeptide chain of other proteins. This proteolytic cleavage is irreversible in cells, and an important type of cellular regulation. Although proteases are one of the earliest and best studied class of enzyme, rhomboids belong to a much more recently discovered type: the intramembrane proteases. What is unique about intramembrane proteases is that their active sites are buried in the lipid bilayer of cell membranes, and they cleave other transmembrane proteins within their transmembrane domains.[2] About 30% of all proteins have transmembrane domains, and their regulated processing often has major biological consequences. Accordingly, rhomboids regulate many important cellular processes, and may be involved in a wide range of human diseases.
- ^ Vinothkumar KR, Pierrat OA, Large JM, Freeman M (June 2013). "Structure of rhomboid protease in complex with β-lactam inhibitors defines the S2' cavity". Structure. 21 (6): 1051–8. doi:10.1016/j.str.2013.03.013. PMC 3690538. PMID 23665170.
- ^ Brown MS, Ye J, Rawson RB, Goldstein JL (February 2000). "Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans". Cell. 100 (4): 391–8. doi:10.1016/S0092-8674(00)80675-3. PMID 10693756. S2CID 12194770.