 | |
| Clinical data | |
|---|---|
| Other names | TZV, Triazavirin |
| ATC code |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.217.074 |
| Chemical and physical data | |
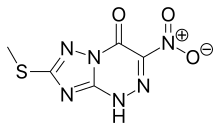
| Formula | C5H4N6O3S |
| Molar mass | 228.19 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Riamilovir is a broad-spectrum antiviral drug developed in Russia through a joint effort of Ural Federal University, Russian Academy of Sciences, Ural Center for Biopharma Technologies and Medsintez Pharmaceutical.[1] It has a novel triazolotriazine core, which represents a new structural class of non-nucleoside antiviral drugs.[2]
The principal action of triazavirin is to inhibit the synthesis of viral ribonucleic acid (RNA) and the replication of viral genomic fragments through its synthetic analogue to the bases of purine nucleosides.[3][4][5]
- ^ "Triazaverin Is Officially Recommended". www.medsintez.com. Retrieved 2021-02-25.
- ^ Rusinov VL, Sapozhnikova IM, Ulomskii EN, Medvedeva NR, Egorov VV, Kiselev OI, et al. (2015). "Nucleophilic substitution of nitro group in nitrotriazolotriazines as a model of potential interaction with cysteine-containing proteins". Chemistry of Heterocyclic Compounds. 51 (3): 275–280. doi:10.1007/s10593-015-1695-4. S2CID 83702396.
- ^ Cite error: The named reference
pmid20194696was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid25051708was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid19275052was invoked but never defined (see the help page).