 | |
| Clinical data | |
|---|---|
| Trade names | Mycobutin[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a693009 |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 85% |
| Protein binding | 85% |
| Metabolism | Liver |
| Elimination half-life | 28 to 62 hours (mean) |
| Excretion | Kidney and fecal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.133.627 |
| Chemical and physical data | |
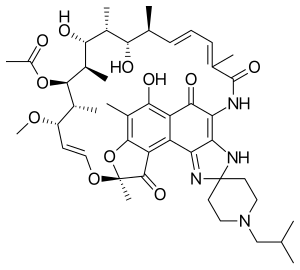
| Formula | C46H62N4O11 |
| Molar mass | 847.019 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Rifabutin (Rfb) is an antibiotic used to treat tuberculosis and prevent and treat Mycobacterium avium complex.[1] It is typically only used in those who cannot tolerate rifampin such as people with HIV/AIDS on antiretrovirals.[1] For active tuberculosis it is used with other antimycobacterial medications.[1] For latent tuberculosis it may be used by itself when the exposure was with drug-resistant TB.[1]
Rifabutin was approved for medical use in the United States in 1992.[1] It is on the World Health Organization's List of Essential Medicines.[3]
- ^ a b c d e f "Rifabutin". The American Society of Health-System Pharmacists. Archived from the original on 20 December 2016. Retrieved 8 December 2016.
- ^ "Product monograph brand safety updates". Health Canada. February 2024. Retrieved 24 March 2024.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.