 | |
| Clinical data | |
|---|---|
| Trade names | Priftin |
| Other names | 3{[(4-cyclopentyl-1-piperazinyl)imino]methyl}rifamycin |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a616011 |
| License data |
|
| Routes of administration | By mouth |
| Drug class | Macrolactam |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | increases when administered with food |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.057.021 |
| Chemical and physical data | |
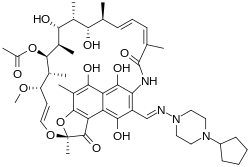
| Formula | C47H64N4O12 |
| Molar mass | 877.045 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 179 to 180 °C (354 to 356 °F) |
| |
| |
| (verify) | |
Rifapentine, sold under the brand name Priftin, is an antibiotic used in the treatment of tuberculosis.[2] In active tuberculosis it is used together with other antituberculosis medications.[2] In latent tuberculosis it is typically used with isoniazid.[2] It is taken by mouth.[2]
Common side effects include low neutrophil counts in the blood, elevated liver enzymes, and white blood cells in the urine.[3] Serious side effects may include liver problems or Clostridioides difficile associated diarrhea.[3] It is unclear if use during pregnancy is safe.[3] Rifapentine is in the rifamycin family of medication and works by blocking DNA-dependent RNA polymerase.[3]
Rifapentine was approved for medical use in the United States in 1998.[2] It is on the World Health Organization's List of Essential Medicines.[4] In many areas of the world it is not easy to get as of 2015[update].[5]
- ^ "Rifapentine (Priftin) Use During Pregnancy". Drugs.com. 2 December 2019. Retrieved 16 March 2020.
- ^ a b c d e "Priftin- rifapentine tablet, film coated". DailyMed. 22 October 2019. Retrieved 6 November 2020.
- ^ a b c d "Rifapentine". The American Society of Health-System Pharmacists. Archived from the original on 20 December 2016. Retrieved 8 December 2016.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Nieburg P, Dubovi T, Angelo S (2015). Tuberculosis—A Complex Health Threat: A Policy Primer of Global TB Challenges. Rowman & Littlefield. p. 15. ISBN 9781442240957. Archived from the original on 2016-12-20.