 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Xifaxan, Zaxine, Xifaxanta, Normix, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604027 |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | < 0.4% |
| Metabolism | Liver |
| Elimination half-life | 6 hours |
| Excretion | Fecal (97%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.111.624 |
| Chemical and physical data | |
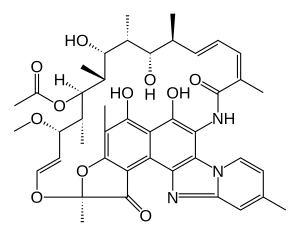
| Formula | C43H51N3O11 |
| Molar mass | 785.891 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 200 to 205 °C (392 to 401 °F) (dec.) |
| |
| |
| | |
Rifaximin, is a non-absorbable, broad spectrum antibiotic mainly used to treat travelers' diarrhea. It is based on the rifamycin antibiotics family. Since its approval in Italy in 1987, it has been licensed in over more than 30 countries for the treatment of a variety of gastrointestinal diseases like irritable bowel syndrome, and hepatic encephalopathy. It acts by inhibiting RNA synthesis in susceptible bacteria by binding to the RNA polymerase enzyme. This binding blocks translocation, which stops transcription.[4] It is marketed under the brand name Xifaxan by Salix Pharmaceuticals.[5]
- ^ "Rifaximin international". Drugs.com. 2 November 2020. Retrieved 11 November 2020.
- ^ "Product monograph brand safety updates". Health Canada. February 2024. Retrieved 24 March 2024.
- ^ "Xifaxan- rifaximin tablet". DailyMed. 1 October 2019. Retrieved 11 November 2020.
- ^ Koo HL, DuPont HL (January 2010). "Rifaximin: a unique gastrointestinal-selective antibiotic for enteric diseases". Current Opinion in Gastroenterology. 26 (1): 17–25. doi:10.1097/MOG.0b013e328333dc8d. PMC 4737517. PMID 19881343.
- ^ "Rifaximin". go.drugbank.com. Retrieved 26 June 2022.